Temperature triggered self-assembly of polypeptides into multivalent spherical micelles
- PMID: 18085778
- PMCID: PMC2855373
- DOI: 10.1021/ja0764862
Temperature triggered self-assembly of polypeptides into multivalent spherical micelles
Abstract
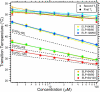
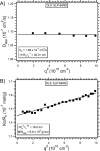
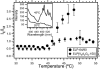
We report herein thermally responsive elastin-like polypeptides (ELPs) in a linear AB diblock architecture with an N-terminal peptide ligand that self-assemble into spherical micelles when heated slightly above body temperature. A series of 10 ELP block copolymers (ELP(BC)'s ) with different molecular weights and hydrophilic-to-hydrophobic block ratios were genetically synthesized by recursive directional ligation. The self-assembly of these polymers from unimers into micelles was investigated by light scattering, fluorescence spectroscopy, and cryo-TEM. These ELP(BC)'s undergo two phase transitions as a function of solution temperature: a unimer-to-spherical micelle transition at an intermediate temperature and a micelle-to-bulk aggregate transition at a higher temperature when the hydrophilic-to-hydrophobic block ratio is between 1:2 and 2:1. The critical micelle temperature is controlled by the length of the hydrophobic block, and the size of the micelle is controlled by both the total ELP(BC) length and hydrophilic-to-hydrophobic block ratio. These polypeptide micelles display a critical micelle concentration in the range 4-8 microM demonstrating the high stability of these structures. These studies have also identified a subset of ELP(BC)'s bearing terminal peptide ligands that are capable of forming multivalent spherical micelles that present multiple copies of the ligand on their corona in the clinically relevant temperature range 37-42 degrees C and target cancer cells. These ELP(BC)'s may be useful for drug targeting by thermally triggered multivalency. More broadly, the design rules uncovered by this study should be applicable to the design of other thermally reversible nanoparticles for diverse applications in medicine and biology.
Figures







References
-
- Whitesides GM, Mathias JP, Seto CT. Science. 1991;254:1312–1319. - PubMed
-
- Rodriguez-Hernandez J, Checot F, Gnanou Y, Lecommandoux S. Prog. Polym. Sci. 2005;30:691–724.
-
- Riess G. Prog. Polym. Sci. 2003;28:1107–1170.
-
- Forster S, Plantenberg T. Angew. Chem.-Int. Edit. 2002;41:689–714. - PubMed
-
- Thomas EL. Science. 1999;286:1307–1307.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

