ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus
- PMID: 18085824
- PMCID: PMC2134958
- DOI: 10.1371/journal.ppat.0030194
ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus
Abstract
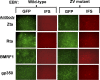
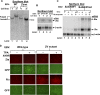
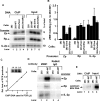
The immediate-early (IE) BZLF1 gene of Epstein-Barr virus (EBV) regulates the switch between latent and lytic infection by EBV. We previously showed that the cellular transcription factor ZEB1 binds to a sequence element, ZV, located at nt -17 to -12 relative to the transcription initiation site of the BZLF1 promoter, Zp, repressing transcription from Zp in a transient transfection assay. Here, we report the phenotype in the context of a whole EBV genome of a variant of EBV strain B95.8 containing a 2-bp substitution mutation in the ZV element of Zp that reduced, but did not eliminate, ZEB1 binding to Zp. Strikingly, epithelial 293 cells latently infected with the EBV ZV mutant spontaneously produced IE-, early-, and late-gene products and infectious virus, while wild-type (WT)-infected 293 cells did not and have never been reported to do so. Furthermore, treatment with the chemical inducers sodium butyrate and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) led to an additional order-of-magnitude production of infectious virus in the ZV mutant-infected 293 cells, but still no virus in the WT-infected 293 cells. Similarly, ZV mutant-infected Burkitt's lymphoma BJAB cells accumulated at least 10-fold more EBV IE mRNAs than did WT-infected BJAB cells, with TPA or sodium butyrate treatment leading to an additional 5- to 10-fold accumulation of EBV IE mRNAs in the ZV mutant-infected cells. Thus, we conclude that ZEB1 binding to Zp plays a central role in regulating the latent-lytic switch in EBV-infected epithelial and B cells, suggesting ZEB1 as a target for lytic-induction therapies in EBV-associated malignancies.
Conflict of interest statement
Figures



Similar articles
-
Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner.J Virol. 2010 Jun;84(12):6139-52. doi: 10.1128/JVI.02706-09. Epub 2010 Apr 7. J Virol. 2010. PMID: 20375168 Free PMC article.
-
Shutoff of BZLF1 gene expression is necessary for immortalization of primary B cells by Epstein-Barr virus.J Virol. 2012 Aug;86(15):8086-96. doi: 10.1128/JVI.00234-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623769 Free PMC article.
-
Zinc finger E-box binding factor 1 plays a central role in regulating Epstein-Barr virus (EBV) latent-lytic switch and acts as a therapeutic target in EBV-associated gastric cancer.Cancer. 2012 Feb 15;118(4):924-36. doi: 10.1002/cncr.26184. Epub 2011 Jun 29. Cancer. 2012. PMID: 21717425
-
Switching of EBV cycles between latent and lytic states.Rev Med Virol. 2014 May;24(3):142-53. doi: 10.1002/rmv.1780. Epub 2013 Dec 11. Rev Med Virol. 2014. PMID: 24339346 Review.
-
Latent and lytic Epstein-Barr virus replication strategies.Rev Med Virol. 2005 Jan-Feb;15(1):3-15. doi: 10.1002/rmv.441. Rev Med Virol. 2005. PMID: 15386591 Review.
Cited by
-
Epigenetic modification of the Epstein-Barr virus BZLF1 promoter regulates viral reactivation from latency.Front Genet. 2013 Apr 9;4:53. doi: 10.3389/fgene.2013.00053. eCollection 2013. Front Genet. 2013. PMID: 23577022 Free PMC article.
-
Differential expression of the miR-200 family microRNAs in epithelial and B cells and regulation of Epstein-Barr virus reactivation by the miR-200 family member miR-429.J Virol. 2010 Aug;84(15):7892-7. doi: 10.1128/JVI.00379-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484493 Free PMC article.
-
EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription.PLoS Pathog. 2011 Nov;7(11):e1002376. doi: 10.1371/journal.ppat.1002376. Epub 2011 Nov 10. PLoS Pathog. 2011. PMID: 22102817 Free PMC article.
-
Histone deacetylases and the nuclear receptor corepressor regulate lytic-latent switch gene 50 in murine gammaherpesvirus 68-infected macrophages.J Virol. 2010 Nov;84(22):12039-47. doi: 10.1128/JVI.00396-10. Epub 2010 Aug 18. J Virol. 2010. PMID: 20719946 Free PMC article.
-
Valpromide Inhibits Lytic Cycle Reactivation of Epstein-Barr Virus.mBio. 2016 Mar 1;7(2):e00113. doi: 10.1128/mBio.00113-16. mBio. 2016. PMID: 26933051 Free PMC article.
References
-
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields' virology. 5th edition. Lippincott, Williams and Wilkins; 2007. pp. 2656–2700.
-
- Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields' virology. 5th edition. Lippincott, Williams and Wilkins; 2007. pp. 2603–2654.
-
- Israel BF, Kenney SC. EBV lytic infection. In: Robertson ES, editor. Epstein-Barr virus. Caister Academic Press; 2005. pp. 571–611.
-
- Speck SH, Chatila T, Flemington E. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 1997;5:399–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

