Cholesterol retention in Alzheimer's brain is responsible for high beta- and gamma-secretase activities and Abeta production
- PMID: 18086530
- PMCID: PMC2720683
- DOI: 10.1016/j.nbd.2007.10.005
Cholesterol retention in Alzheimer's brain is responsible for high beta- and gamma-secretase activities and Abeta production
Abstract
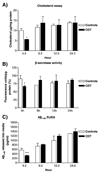
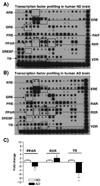
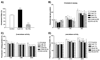
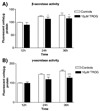
Alzheimer's disease (AD) is characterized by overproduction of A beta derived from APP cleavage via beta- and gamma-secretase pathway. Recent evidence has linked altered cholesterol metabolism to AD pathogenesis. In this study, we show that AD brain had significant cholesterol retention and high beta- and gamma-secretase activities as compared to age-matched non-demented controls (ND). Over one-half of AD patients had an apoE4 allele but none of the ND. beta- and gamma-secretase activities were significantly stimulated in vitro by 40 and 80 microM cholesterol in AD and ND brains, respectively. Both secretase activities in AD brain were more sensitive to cholesterol (40 microM) than those of ND (80 microM). Filipin-stained cholesterol overlapped with BACE and A beta in AD brain sections. Cholesterol (10-80 microM) added to N2a cultures significantly increased cellular cholesterol, beta- and gamma-secretase activities and A beta secretion. Similarly, addition of cholesterol (20-80 microM) to cell lysates stimulated both in vitro secretase activities. Ergosterol slightly decreased beta-secretase activity at 20-80 microM, but strongly inhibited gamma-secretase activity at 40 microM. Cholesterol depletion reduced cellular cholesterol, beta-secretase activity and A beta secretion. Transcription factor profiling shows that several key nuclear receptors involving cholesterol metabolism were significantly altered in AD brain, including decreased LXR-beta, PPAR and TR, and increased RXR. Treatment of N2a cells with LXR, RXR or PPAR agonists strongly stimulated cellular cholesterol efflux to HDL and reduced cellular cholesterol and beta-/gamma-secretase activities. This study provides direct evidence that cholesterol homeostasis is impaired in AD brain and suggests that altered levels or activities of nuclear receptors may contribute to cholesterol retention which likely enhances beta- and gamma-secretase activities and A beta production in human brain.
Figures







References
-
- Abulrob A, Tauskela JS, Mealing G, Brunette E, Faid K, Stanimirovic D. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-D-aspartate receptor redistribution. J. Neurochem. 2005;92(6):1477–1486. - PubMed
-
- Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB, Jr, Gonzalez FJ. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 2002;22(8):2607–2619. - PMC - PubMed
-
- Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

