Tetrameric restriction enzymes: expansion to the GIY-YIG nuclease family
- PMID: 18086711
- PMCID: PMC2241918
- DOI: 10.1093/nar/gkm1090
Tetrameric restriction enzymes: expansion to the GIY-YIG nuclease family
Abstract
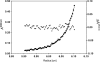
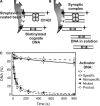
The GIY-YIG nuclease domain was originally identified in homing endonucleases and enzymes involved in DNA repair and recombination. Many of the GIY-YIG family enzymes are functional as monomers. We show here that the Cfr42I restriction endonuclease which belongs to the GIY-YIG family and recognizes the symmetric sequence 5'-CCGC/GG-3' ('/' indicates the cleavage site) is a tetramer in solution. Moreover, biochemical and kinetic studies provided here demonstrate that the Cfr42I tetramer is catalytically active only upon simultaneous binding of two copies of its recognition sequence. In that respect Cfr42I resembles the homotetrameric Type IIF restriction enzymes that belong to the distinct PD-(E/D)XK nuclease superfamily. Unlike the PD-(E/D)XK enzymes, the GIY-YIG nuclease Cfr42I accommodates an extremely wide selection of metal-ion cofactors, including Mg2+, Mn2+, Co2+, Zn2+, Ni2+, Cu2+ and Ca2+. To our knowledge, Cfr42I is the first tetrameric GIY-YIG family enzyme. Similar structural arrangement and phenotypes displayed by restriction enzymes of the PD-(E/D)XK and GIY-YIG nuclease families point to the functional significance of tetramerization.
Figures
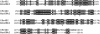




References
-
- Sapranauskas R, Sasnauskas G, Lagunavicius A, Vilkaitis G, Lubys A, Siksnys V. Novel subtype of type IIs restriction enzymes. BfiI endonuclease exhibits similarities to the EDTA-resistant nuclease Nuc of Salmonella typhimurium. J. Biol. Chem. 2000;275:30878–30885. - PubMed
-
- Bujnicki JM, Radlinska M, Rychlewski L. Polyphyletic evolution of type II restriction enzymes revisited: two independent sources of second-hand folds revealed. Trends Biochem. Sci. 2001;26:9–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

