Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial
- PMID: 18086727
- PMCID: PMC2569144
- DOI: 10.1136/ard.2007.085084
Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial
Abstract
Objective: Assess the effect of abatacept on progression of structural damage over 2 years in patients with rheumatoid arthritis who had an inadequate response to methotrexate.
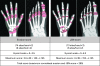
Methods: 539 patients entered an open-label extension of the AIM (Abatacept in Inadequate responders to Methotrexate) trial and received abatacept. Radiographic assessment of the hands and feet was performed at baseline, year 1 and year 2. At year 2, each patient's radiographs were scored for progression blinded to sequence and treatment allocation.
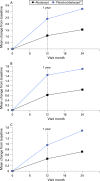
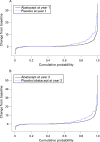
Results: In patients treated with abatacept for 2 years, greater reduction in progression of structural damage was observed in year 2 than in year 1. The mean change in total Genant-modified Sharp scores was reduced from 1.07 units in year 1 to 0.46 units in year 2. Similar reductions were observed in erosion and joint space narrowing scores. Following 2 years of treatment with abatacept, 50% of patients had no progression of structural damage as defined by a change in the total score of < or =0 compared with baseline. 56% of patients treated with abatacept had no progression during the first year compared with 45% of patients treated with placebo. In their second year of treatment with abatacept, more patients had no progression than in the first year (66% vs 56%).
Conclusions: Abatacept has a sustained effect that inhibits progression of structural damage. Furthermore, the mean change in radiographic progression in patients treated with abatacept for 2 years was significantly lower in year 2 versus year 1, suggesting that abatacept may have an increasing disease-modifying effect on structural damage over time.
Conflict of interest statement
Figures
References
-
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001;344:907–16 - PubMed
-
- Goronzy JJ, Weyand CM. T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol 2004;16:212–17 - PubMed
-
- Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, et al. Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum 2002;46:1470–9 - PubMed
-
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 2005;353:1114–23 - PubMed
-
- Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 2006;144:865–76 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

