Adsorption of amikacin, a significant mechanism of elimination by hemofiltration
- PMID: 18086842
- PMCID: PMC2258534
- DOI: 10.1128/AAC.00858-07
Adsorption of amikacin, a significant mechanism of elimination by hemofiltration
Abstract
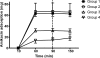
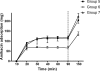
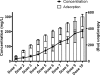
We used an in vitro model of continuous venovenous hemofiltration (CVVH) to characterize amikacin adsorption by polyacrylonitrile (PAN) and polyamide filters. A blood-crystalloid mixture dosed with amikacin was pumped from a reservoir through a hemofiltration circuit and back to the reservoir. All ultrafiltrate was also returned to the reservoir. The level of adsorption was calculated from the fall in the amikacin concentration. The dose and the initial concentration of amikacin were varied, as were the pH, the type of hemofilter, and the hemofilter surface area. The reversibility of adsorption and the effect of repeated dosing were also studied. The level of adsorption by 0.6-m2 PAN filters was significantly greater than that by 0.6-m2 polyamide filters. Adsorption was increased by increasing the dose of amikacin even when the initial concentration was unchanged. It was unaffected by the pH (pH 6.8 or 7.4) or the hemofilter surface area (0.6 m2 or 0.9 m2). Repeated doses of amikacin resulted in further adsorption. In a saturation experiment, the maximum adsorptive capacity of 0.6-m2 PAN hemofilters was at least 546.9 mg (range, 427.6 to 577.5 mg). The adsorption of amikacin by hemofilters is irreversible and was associated with the dose and the hemofilter material but not the hemofilter surface area. Close monitoring of peak amikacin levels should be considered for patients receiving CVVH with PAN hemofilters.
Figures

References
-
- Bartal, C., A. Danon, F. Schlaeffer, K. Reisenberg, M. Alkan, R. Smoliakov, A. Sidi, and Y. Almog. 2003. Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am. J. Med. 114:194-198. - PubMed
-
- Bressolle, F., J. M. Kinowski, J. E. de la Coussaye, N. Wynn, J. J. Eledjam, and M. Galtier. 1994. Clinical pharmacokinetics during continuous haemofiltration. Clin. Pharmacokinet. 26:457-471. - PubMed
-
- Choi, G., C. D. Gomersall, J. Lipman, A. Wong, G. M. Joynt, P. Leung, S. J. Ramsay, and O. M. Ho. 2004. The effect of adsorption, filter material and point of dilution on antibiotic elimination by haemofiltration: an in vitro study of levofloxacin. Int. J. Antimicrob. Agents 24:468-472. - PubMed
-
- Cigarran-Guldris, S., M. E. Brier, and T. A. Golper. 1991. Tobramycin clearance during simulated continuous arteriovenous hemodialysis. Contrib. Nephrol. 93:120-123. - PubMed
-
- Clark, W. R., W. L. Macias, B. A. Molitoris, and N. H. Wang. 1994. Membrane adsorption of beta 2-microglobulin: equilibrium and kinetic characterization. Kidney Int. 46:1140-1146. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

