Talin is required for integrin-mediated platelet function in hemostasis and thrombosis
- PMID: 18086863
- PMCID: PMC2150986
- DOI: 10.1084/jem.20071800
Talin is required for integrin-mediated platelet function in hemostasis and thrombosis
Abstract
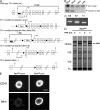
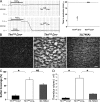
Integrins are critical for hemostasis and thrombosis because they mediate both platelet adhesion and aggregation. Talin is an integrin-binding cytoplasmic adaptor that is a central organizer of focal adhesions, and loss of talin phenocopies integrin deletion in Drosophila. Here, we have examined the role of talin in mammalian integrin function in vivo by selectively disrupting the talin1 gene in mouse platelet precursor megakaryocytes. Talin null megakaryocytes produced circulating platelets that exhibited normal morphology yet manifested profoundly impaired hemostatic function. Specifically, platelet-specific deletion of talin1 led to spontaneous hemorrhage and pathological bleeding. Ex vivo and in vitro studies revealed that loss of talin1 resulted in dramatically impaired integrin alphaIIbbeta3-mediated platelet aggregation and beta1 integrin-mediated platelet adhesion. Furthermore, loss of talin1 strongly inhibited the activation of platelet beta1 and beta3 integrins in response to platelet agonists. These data establish that platelet talin plays a crucial role in hemostasis and provide the first proof that talin is required for the activation and function of mammalian alpha2beta1 and alphaIIbbeta3 integrins in vivo.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

