Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo
- PMID: 18086864
- PMCID: PMC2150972
- DOI: 10.1084/jem.20071827
Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo
Abstract
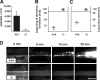
Platelet adhesion and aggregation at sites of vascular injury are essential for normal hemostasis but may also lead to pathological thrombus formation, causing diseases such as myocardial infarction or stroke. Heterodimeric receptors of the integrin family play a central role in the adhesion and aggregation of platelets. In resting platelets, integrins exhibit a low affinity state for their ligands, and they shift to a high affinity state at sites of vascular injury. It has been proposed that direct binding of the cytoskeletal protein talin1 to the cytoplasmic domain of the integrin beta subunits is necessary and sufficient to trigger the activation of integrins to this high affinity state, but direct in vivo evidence in support of this hypothesis is still lacking. Here, we show that platelets from mice lacking talin1 are unable to activate integrins in response to all known major platelet agonists while other cellular functions are still preserved. As a consequence, mice with talin-deficient platelets display a severe hemostatic defect and are completely resistant to arterial thrombosis. Collectively, these experiments demonstrate that talin is required for inside-out activation of platelet integrins in hemostasis and thrombosis.
Figures

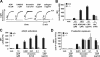

References
-
- Ruggeri, Z.M. 2002. Platelets in atherothrombosis. Nat. Med. 8:1227–1234. - PubMed
-
- Nieswandt, B., and S.P. Watson. 2003. Platelet-collagen interaction: is GPVI the central receptor? Blood. 102:449–461. - PubMed
-
- Shattil, S.J., H. Kashiwagi, and N. Pampori. 1998. Integrin signaling: the platelet paradigm. Blood. 91:2645–2657. - PubMed
-
- Hynes, R.O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

