Incorporation of the noncoding roX RNAs alters the chromatin-binding specificity of the Drosophila MSL1/MSL2 complex
- PMID: 18086881
- PMCID: PMC2258739
- DOI: 10.1128/MCB.00910-07
Incorporation of the noncoding roX RNAs alters the chromatin-binding specificity of the Drosophila MSL1/MSL2 complex
Erratum in
- Mol Cell Biol. 2008 Apr;28(8):2850
Abstract
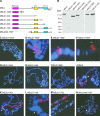
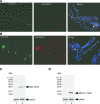
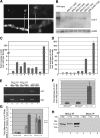
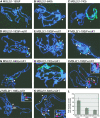
The male-specific lethal (MSL) protein-RNA complex is required for X chromosome dosage compensation in Drosophila melanogaster. The MSL2 and MSL1 proteins form a complex and are essential for X chromosome binding. In addition, the MSL complex must integrate at least one of the noncoding roX RNAs for normal X chromosome binding. Here we find the amino-terminal RING finger domain of MSL2 binds as a complex with MSL1 to the heterochromatic chromocenter and a few sites on the chromosome arms. This binding required the same amino-terminal basic motif of MSL1 previously shown to be essential for binding to high-affinity sites on the X chromosome. While the RING finger domain of MSL2 is sufficient to increase the expression of roX1 in females, activation of roX2 requires motifs in the carboxyl-terminal domain. Binding to hundreds of sites on the X chromosome and efficient incorporation of the roX RNAs into the MSL complex require proline-rich and basic motifs in the carboxyl-terminal domain of MSL2. We suggest that incorporation of the roX RNAs into the MSL complex alters the binding specificity of the chromatin-binding module formed by the amino-terminal domains of MSL1 and MSL2.
Figures




Similar articles
-
N-terminus of Drosophila melanogaster MSL1 is critical for dosage compensation.Elife. 2024 Dec 19;13:RP93241. doi: 10.7554/eLife.93241. Elife. 2024. PMID: 39699942 Free PMC article.
-
The amino-terminal region of Drosophila MSL1 contains basic, glycine-rich, and leucine zipper-like motifs that promote X chromosome binding, self-association, and MSL2 binding, respectively.Mol Cell Biol. 2005 Oct;25(20):8913-24. doi: 10.1128/MCB.25.20.8913-8924.2005. Mol Cell Biol. 2005. PMID: 16199870 Free PMC article.
-
Autoregulation of the Drosophila Noncoding roX1 RNA Gene.PLoS Genet. 2012;8(3):e1002564. doi: 10.1371/journal.pgen.1002564. Epub 2012 Mar 15. PLoS Genet. 2012. PMID: 22438819 Free PMC article.
-
The right dose for every sex.Chromosoma. 2007 Apr;116(2):95-106. doi: 10.1007/s00412-006-0089-x. Epub 2006 Nov 24. Chromosoma. 2007. PMID: 17124606 Free PMC article. Review.
-
Dosage Compensation of the X Chromosome: A Complex Epigenetic Assignment Involving Chromatin Regulators and Long Noncoding RNAs.Annu Rev Biochem. 2018 Jun 20;87:323-350. doi: 10.1146/annurev-biochem-062917-011816. Epub 2018 Apr 18. Annu Rev Biochem. 2018. PMID: 29668306 Review.
Cited by
-
Cell-free genomics reveal intrinsic, cooperative and competitive determinants of chromatin interactions.Nucleic Acids Res. 2021 Jul 21;49(13):7602-7617. doi: 10.1093/nar/gkab558. Nucleic Acids Res. 2021. PMID: 34181732 Free PMC article.
-
Divergent evolution toward sex chromosome-specific gene regulation in Drosophila.Genes Dev. 2021 Jul 1;35(13-14):1055-1070. doi: 10.1101/gad.348411.121. Epub 2021 Jun 17. Genes Dev. 2021. PMID: 34140353 Free PMC article.
-
Requirement of male-specific dosage compensation in Drosophila females--implications of early X chromosome gene expression.PLoS Genet. 2010 Jul 29;6(7):e1001041. doi: 10.1371/journal.pgen.1001041. PLoS Genet. 2010. PMID: 20686653 Free PMC article.
-
A new player in X identification: the CLAMP protein is a key factor in Drosophila dosage compensation.Chromosome Res. 2014 Dec;22(4):505-15. doi: 10.1007/s10577-014-9438-4. Epub 2014 Aug 8. Chromosome Res. 2014. PMID: 25102930 Free PMC article. Review.
-
A mutually exclusive stem-loop arrangement in roX2 RNA is essential for X-chromosome regulation in Drosophila.Genes Dev. 2017 Oct 1;31(19):1973-1987. doi: 10.1101/gad.304600.117. Epub 2017 Oct 24. Genes Dev. 2017. PMID: 29066499 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
