Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy
- PMID: 18086901
- PMCID: PMC3418347
- DOI: 10.2337/db07-1441
Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy
Abstract
Objective: The key pancreatic transcription factor pancreatic duodenal homeobox-1 (Pdx1), known to control development and maintenance of pancreatic beta-cells, possesses a protein transduction domain (PTD) that facilitates its entry into cells. We therefore sought to evaluate the capacity of in vivo-administered recombinant Pdx1 (rPdx1) to ameliorate hyperglycemia in mice with streptozotocin-induced diabetes.
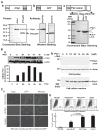
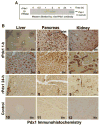
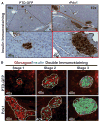
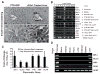
Research design and methods: Cell entry and transcriptional regulatory properties of rPdx1 protein and its PTD-deletion mutant rPdx1Delta protein, as well as a PTD-green fluorescent protein, were evaluated in vitro. After intraperitoneal rPdx1 injection into mice with streptozotocin-induced diabetes, we assessed its action on blood glucose levels, insulin content, intraperitoneal glucose tolerance test (IPGTT), Pdx1 distribution, pancreatic gene expression, islet cell proliferation, and organ histology.
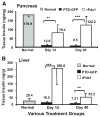
Results: Restoration of euglycemia in Pdx1-treated diabetic mice was evident by improved IPGTT and glucose-stimulated insulin release. Insulin, glucagon, and Ki67 immunostaining revealed increased islet cell number and proliferation in pancreata of rPdx1-treated mice. Real-time PCR of pancreas and liver demonstrated upregulation of INS and PDX1 genes and other genes relevant to pancreas regeneration. While the time course of beta-cell gene expression and serum/tissue insulin levels indicated that both liver- and pancreas-derived insulin contributed to restoration of normoglycemia, near-total pancreatectomy resulted in hyperglycemia, suggesting that beta-cell regeneration played the primary role in rPdx1-induced glucose homeostasis.
Conclusions: rPdx1 treatment of mice with streptozotocin-induced diabetes promotes beta-cell regeneration and liver cell reprogramming, leading to restoration of normoglycemia. This novel PTD-based protein therapy offers a promising way to treat patients with diabetes while avoiding potential side effects associated with the use of viral vectors.
Figures


Similar articles
-
Adult rat liver cells transdifferentiated with lentiviral IPF1 vectors reverse diabetes in mice: an ex vivo gene therapy approach.Diabetologia. 2007 Jan;50(1):121-30. doi: 10.1007/s00125-006-0509-8. Epub 2006 Nov 28. Diabetologia. 2007. PMID: 17131142
-
Reversal of streptozotocin-induced diabetes in rats by gene therapy with betacellulin and pancreatic duodenal homeobox-1.Gene Ther. 2007 Jul;14(14):1102-10. doi: 10.1038/sj.gt.3302963. Epub 2007 Apr 26. Gene Ther. 2007. PMID: 17460716
-
Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice.Int J Dev Biol. 2010;54(4):699-705. doi: 10.1387/ijdb.092953hk. Int J Dev Biol. 2010. PMID: 19757377
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
-
A feat of metabolic proportions: Pdx1 orchestrates islet development and function in the maintenance of glucose homeostasis.Mol Genet Metab. 2007 Sep-Oct;92(1-2):43-55. doi: 10.1016/j.ymgme.2007.06.008. Epub 2007 Jul 30. Mol Genet Metab. 2007. PMID: 17659992 Free PMC article. Review.
Cited by
-
PCSK9 Contributes to the Cholesterol, Glucose, and Insulin2 Homeostasis in Seminiferous Tubules and Maintenance of Immunotolerance in Testis.Front Cell Dev Biol. 2022 May 2;10:889972. doi: 10.3389/fcell.2022.889972. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35586340 Free PMC article.
-
Pdx-1 or Pdx-1-VP16 protein transduction induces beta-cell gene expression in liver-stem WB cells.BMC Res Notes. 2009 Jan 9;2:3. doi: 10.1186/1756-0500-2-3. BMC Res Notes. 2009. PMID: 19134185 Free PMC article.
-
Effects of Biotin Supplementation During the First Week Postweaning Increases Pancreatic Islet Area, Beta-Cell Proportion, Islets Number, and Beta-Cell Proliferation.J Med Food. 2018 Mar;21(3):274-281. doi: 10.1089/jmf.2017.0077. Epub 2017 Oct 25. J Med Food. 2018. PMID: 29068758 Free PMC article.
-
Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes.Lab Invest. 2010 Jan;90(1):31-9. doi: 10.1038/labinvest.2009.116. Epub 2009 Nov 9. Lab Invest. 2010. PMID: 19901909 Free PMC article.
-
Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells.Transplantation. 2010 Mar 27;89(6):677-85. doi: 10.1097/TP.0b013e3181c9dc7d. Transplantation. 2010. PMID: 20110858 Free PMC article.
References
-
- Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. - PubMed
-
- Meier JJ, Bhushan A, Butler PC. The potential for stem cell therapy in diabetes. Pediatr Res. 2006;59:65R–73R. - PubMed
-
- Trucco M. Is facilitating pancreatic beta cell regeneration a valid option for clinical therapy? Cell Transplant. 2006;15(Suppl. 1):S75–S84. - PubMed
-
- Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–4605. - PubMed
-
- Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

