Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone
- PMID: 18086919
- PMCID: PMC2140044
- DOI: 10.1083/jcb.200702031
Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone
Abstract
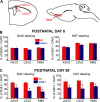
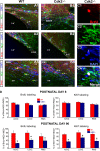
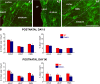
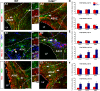
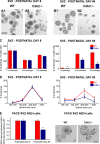
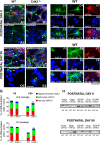
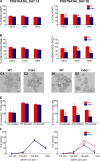
We investigated the function of cyclin-dependent kinase 2 (Cdk2) in neural progenitor cells during postnatal development. Chondroitin sulfate proteoglycan (NG2)-expressing progenitor cells of the subventricular zone (SVZ) show no significant difference in density and proliferation between Cdk2(-/-) and wild-type mice at perinatal ages and are reduced only in adult Cdk2(-/-) mice. Adult Cdk2(-/-) SVZ cells in culture display decreased self-renewal capacity and enhanced differentiation. Compensatory mechanisms in perinatal Cdk2(-/-) SVZ cells, which persist until postnatal day 15, involve increased Cdk4 expression that results in retinoblastoma protein inactivation. A subsequent decline in Cdk4 activity to wild-type levels in postnatal day 28 Cdk2(-/-) cells coincides with lower NG2+ proliferation and self-renewal capacity similar to adult levels. Cdk4 silencing in perinatal Cdk2(-/-) SVZ cells abolishes Cdk4 up-regulation and reduces cell proliferation and self- renewal to adult levels. Conversely, Cdk4 overexpression in adult SVZ cells restores proliferative capacity to wild-type levels. Thus, although Cdk2 is functionally redundant in perinatal SVZ, it is important for adult progenitor cell proliferation and self-renewal through age-dependent regulation of Cdk4.
Figures



References
-
- Anton, E.S., H.T. Ghashghaei, J.L. Weber, C. McCann, T.M. Fischer, I.D. Cheung, M. Gassmann, A. Messing, R. Klein, M.H. Schwab, et al. 2004. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat. Neurosci. 7:1319–1328. - PubMed
-
- Beijersbergen, R.L., L. Carlee, R.M. Kerkhoven, and R. Bernards. 1995. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 9:1340–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

