Staged in vitro reconstitution and implantation of engineered rat kidney tissue
- PMID: 18087037
- PMCID: PMC2409245
- DOI: 10.1073/pnas.0710428105
Staged in vitro reconstitution and implantation of engineered rat kidney tissue
Abstract
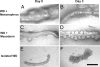
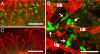
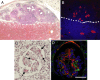
A major hurdle for current xenogenic-based and other approaches aimed at engineering kidney tissues is reproducing the complex three-dimensional structure of the kidney. Here, a stepwise, in vitro method of engineering rat kidney-like tissue capable of being implanted is described. Based on the fact that the stages of kidney development are separable into in vitro modules, an approach was devised that sequentially induces an epithelial tubule (the Wolffian duct) to undergo in vitro budding, followed by branching of a single isolated bud and its recombination with metanephric mesenchyme. Implantation of the recombined tissue results in apparent early vascularization. Thus, in principle, an unbranched epithelial tubular structure (potentially constructed from cultured cells) can be induced to form kidney tissue such that this in vitro engineered tissue is capable of being implanted in host rats and developing glomeruli with evidence of early vascularization. Optimization studies (of growth factor and matrix) indicate multiple suitable combinations and suggest both a most robust and a minimal system. A whole-genome microarray analysis suggested that recombined tissue recapitulated gene expression changes that occur in vivo during later stages of kidney development, and a functional assay demonstrated that the recombined tissue was capable of transport characteristic of the differentiating nephron. The approach includes several points where tissue can be propagated. The data also show how functional, 3D kidney tissue can assemble by means of interactions of independent modules separable in vitro, potentially facilitating systems-level analyses of kidney development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney.Tissue Eng Part A. 2010 Aug;16(8):2441-55. doi: 10.1089/ten.TEA.2009.0548. Tissue Eng Part A. 2010. PMID: 20214453 Free PMC article.
-
A strategy for in vitro propagation of rat nephrons.Kidney Int. 2002 Dec;62(6):1958-65. doi: 10.1046/j.1523-1755.2002.00694.x. Kidney Int. 2002. PMID: 12427120
-
Concise review: can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs?Stem Cells Transl Med. 2013 Dec;2(12):993-1000. doi: 10.5966/sctm.2013-0076. Epub 2013 Nov 4. Stem Cells Transl Med. 2013. PMID: 24191267 Free PMC article. Review.
-
Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys.Sci Rep. 2015 Mar 13;5:9092. doi: 10.1038/srep09092. Sci Rep. 2015. PMID: 25766625 Free PMC article.
-
Development of the tubular nephron.Semin Nephrol. 1995 Jul;15(4):315-26. Semin Nephrol. 1995. PMID: 7569411 Review.
Cited by
-
Physiology assays in human kidney organoids.Am J Physiol Renal Physiol. 2022 Jun 1;322(6):F625-F638. doi: 10.1152/ajprenal.00400.2021. Epub 2022 Apr 4. Am J Physiol Renal Physiol. 2022. PMID: 35379001 Free PMC article. Review.
-
Multidisciplinary approaches for elucidating genetics and molecular pathogenesis of urinary tract malformations.Kidney Int. 2022 Mar;101(3):473-484. doi: 10.1016/j.kint.2021.09.034. Epub 2021 Nov 12. Kidney Int. 2022. PMID: 34780871 Free PMC article. Review.
-
Organogenesis forum lecture: In vitro kidney development, tissue engineering and systems biology.Organogenesis. 2008 Jul;4(3):137-43. doi: 10.4161/org.4.3.6498. Organogenesis. 2008. PMID: 19279725 Free PMC article.
-
The organic anion transporter (OAT) family: a systems biology perspective.Physiol Rev. 2015 Jan;95(1):83-123. doi: 10.1152/physrev.00025.2013. Physiol Rev. 2015. PMID: 25540139 Free PMC article. Review.
-
Engineering the vasculature for islet transplantation.Acta Biomater. 2019 Sep 1;95:131-151. doi: 10.1016/j.actbio.2019.05.051. Epub 2019 May 23. Acta Biomater. 2019. PMID: 31128322 Free PMC article. Review.
References
-
- System USRD. USRDS 2005 Annual Data Report. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), US Department of Health and Human Services (DHHS); 2005.
-
- Humes HD, Weitzel WF, Fissell WH. Renal cell therapy in the treatment of patients with acute and chronic renal failure. Blood Purif. 2004;22:60–72. - PubMed
-
- Rogers SA, Lowell JA, Hammerman NA, Hammerman MR. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54:27–37. - PubMed
-
- Johkura K, Liang Y, Teng RF, Ogiwara N, Sasaki K. Nephrogenesis accompanied by vascularisation in the mouse embryonic metanephros transplanted into the adult kidney for the creation of additional nephrons. Nephrology. 2002;7:92–100.
-
- Dekel B, et al. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9:53–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

