DNA damage-induced cell death: lessons from the central nervous system
- PMID: 18087290
- PMCID: PMC2626635
- DOI: 10.1038/cr.2007.110
DNA damage-induced cell death: lessons from the central nervous system
Abstract
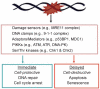
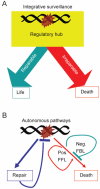
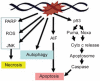
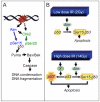
DNA damage can, but does not always, induce cell death. While several pathways linking DNA damage signals to mitochondria-dependent and -independent death machineries have been elucidated, the connectivity of these pathways is subject to regulation by multiple other factors that are not well understood. We have proposed two conceptual models to explain the delayed and variable cell death response to DNA damage: integrative surveillance versus autonomous pathways. In this review, we discuss how these two models may explain the in vivo regulation of cell death induced by ionizing radiation (IR) in the developing central nervous system, where the death response is regulated by radiation dose, cell cycle status and neuronal development.
Figures

Similar articles
-
Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system.Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):889-94. doi: 10.1073/pnas.97.2.889. Proc Natl Acad Sci U S A. 2000. PMID: 10639175 Free PMC article.
-
Ionizing radiation but not anticancer drugs causes cell cycle arrest and failure to activate the mitochondrial death pathway in MCF-7 breast carcinoma cells.Oncogene. 2001 Aug 16;20(36):5043-53. doi: 10.1038/sj.onc.1204659. Oncogene. 2001. PMID: 11526489
-
Significance of p53 dynamics in regulating apoptosis in response to ionizing radiation, and polypharmacological strategies.Sci Rep. 2014 Sep 1;4:6245. doi: 10.1038/srep06245. Sci Rep. 2014. PMID: 25175563 Free PMC article.
-
Retention of γH2AX foci as an indication of lethal DNA damage.Radiother Oncol. 2011 Oct;101(1):18-23. doi: 10.1016/j.radonc.2011.05.055. Epub 2011 Jun 23. Radiother Oncol. 2011. PMID: 21704409 Review.
-
A comprehensive review of sensors of radiation-induced damage, radiation-induced proximal events, and cell death.Immunol Rev. 2025 Jan;329(1):e13409. doi: 10.1111/imr.13409. Epub 2024 Oct 19. Immunol Rev. 2025. PMID: 39425547 Free PMC article. Review.
Cited by
-
The related miRNAs involved in doxorubicin resistance or sensitivity of various cancers: an update.Cancer Chemother Pharmacol. 2021 Nov;88(5):771-793. doi: 10.1007/s00280-021-04337-8. Epub 2021 Sep 12. Cancer Chemother Pharmacol. 2021. PMID: 34510251 Review.
-
Oncolytic Newcastle Disease Virus Co-Delivered with Modified PLGA Nanoparticles Encapsulating Temozolomide against Glioblastoma Cells: Developing an Effective Treatment Strategy.Molecules. 2022 Sep 6;27(18):5757. doi: 10.3390/molecules27185757. Molecules. 2022. PMID: 36144488 Free PMC article.
-
Base Excision Repair of N6-Deoxyadenosine Adducts of 1,3-Butadiene.Biochemistry. 2016 Nov 1;55(43):6070-6081. doi: 10.1021/acs.biochem.6b00553. Epub 2016 Oct 21. Biochemistry. 2016. PMID: 27552084 Free PMC article.
-
Cooling of Cells and Organs Confers Extensive DNA Strand Breaks Through Oxidative Stress and ATP Depletion.Cell Transplant. 2022 Jan-Dec;31:9636897221108705. doi: 10.1177/09636897221108705. Cell Transplant. 2022. PMID: 35808831 Free PMC article.
-
Molecular and cellular pathways associated with chromosome 1p deletions during colon carcinogenesis.Clin Exp Gastroenterol. 2011;4:75-119. doi: 10.2147/CEG.S17114. Epub 2011 May 3. Clin Exp Gastroenterol. 2011. PMID: 21753893 Free PMC article.
References
-
- Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–783. - PubMed
-
- Blank M, Shiloh Y. Programs for cell death: apoptosis is only one way to go. Cell Cycle. 2007;6:686–695. - PubMed
-
- Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–450. - PubMed
-
- Bentle MS, Bey EA, Dong Y, Reinicke KE, Boothman DA. New tricks for old drugs: the anticarcinogenic potential of DNA repair inhibitors. J Mol Histol. 2006;37:203–218. - PubMed
-
- Michod D, Widmann C. DNA-damage sensitizers: potential new therapeutical tools to improve chemotherapy. Crit Rev Oncol Hematol. 2007;63:160–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

