Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors
- PMID: 18087583
- PMCID: PMC2137133
- DOI: 10.1289/ehp.10190
Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors
Abstract
To support the development and implementation of biological monitoring programs, we need quantitative technologies for measuring xenobiotic exposure. Microanalytical based sensors that work with complex biomatrices such as blood, urine, or saliva are being developed and validated and will improve our ability to make definitive associations between chemical exposures and disease. Among toxic metals, lead continues to be one of the most problematic. Despite considerable efforts to identify and eliminate Pb exposure sources, this metal remains a significant health concern, particularly for young children. Ongoing research focuses on the development of portable metal analyzers that have many advantages over current available technologies, thus potentially representing the next generation of toxic metal analyzers. In this article, we highlight the development and validation of two classes of metal analyzers for the voltammetric detection of Pb, including: a) an analyzer based on flow injection analysis and anodic stripping voltammetry at a mercury-film electrode, and b) Hg-free metal analyzers employing adsorptive stripping voltammetry and novel nanostructure materials that include the self-assembled monolayers on mesoporous supports and carbon nanotubes. These sensors have been optimized to detect Pb in urine, blood, and saliva as accurately as the state-of-the-art inductively coupled plasma-mass spectrometry with high reproducibility, and sensitivity allows. These improved and portable analytical sensor platforms will facilitate our ability to conduct biological monitoring programs to understand the relationship between chemical exposure assessment and disease outcomes.
Keywords: biomonitoring; dosimetry technology; electrochemical sensors; exposure assessment; lead (Pb).
Figures

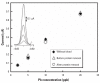

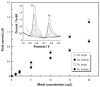


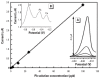

References
-
- Banks EC, Ferretti LE, Shucard DW. Effects of low level of lead exposure on cognitive function in children: a review of behavioral, neuropsychological and biological evidence. Neurotoxicol. 1997;18(1):237–281. - PubMed
-
- Beck BD. An update on exposure and effects of lead. Fundam Appl Toxicol. 1992;18(1):1–16. - PubMed
-
- Bellinger DC. Lead. Pediatrics. 2004;113:1016–1022. - PubMed
-
- Birnbaum JC, Busche B, Lin Y, Shaw WJ, Fryxell GE. Synthesis of carbamoylphosphonate silanes for the selective sequestration of actinides. Chem Commun. 2002;13:1374–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
