Fine-tuning of cell signaling by glypicans
- PMID: 18087675
- PMCID: PMC11114805
- DOI: 10.1007/s00018-007-7471-6
Fine-tuning of cell signaling by glypicans
Abstract
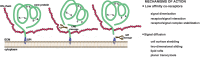
Signaling peptides of the extracellular environment regulate cell biological processes underlying embryonic development, tissue homeostasis, and pathophysiology. The heparan sulphate proteoglycans, glypicans, have evolved as essential modulators of key regulatory proteins such as Wnt, Bmp, Fgf, and Shh. By acting on signal spreading and receptor activation, glypicans can control signal read-out and fate in targeted cells. Genetic and embryological studies have highlighted that glypicans act in a temporal and spatially regulated manner to modulate distinct cellular events. However, alterations of glypican function underlie human congenital malformations and cancer. Recent reports are starting to reveal their mechanism of action and how they can ensure tight modulation of cell signaling.
Figures
References
-
- Nybakken K, Perrimon N. Heparan sulfate proteoglycan modulation of developmental signaling in Dro sophila . Biochim Biophys Acta. 2002;1573:280–291. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

