Structure-activity relationships of cyclic lactam analogues of alpha-melanocyte-stimulating hormone (alpha-MSH) targeting the human melanocortin-3 receptor
- PMID: 18088090
- PMCID: PMC2587288
- DOI: 10.1021/jm070461w
Structure-activity relationships of cyclic lactam analogues of alpha-melanocyte-stimulating hormone (alpha-MSH) targeting the human melanocortin-3 receptor
Abstract
A variety of dicarboxylic acid linkers introduced between the alpha-amino group of Pro(6) and the -amino group of Lys(10) of the cyclic lactam alpha-melanocyte-stimulating hormone (alpha-MSH)-derived Pro(6)-D-Phe(7)/D-Nal(2')(7)-Arg(8)-Trp(9)-Lys(10)-NH2 pentapeptide template lead to nanomolar range and selective hMC3R agonists and antagonists. Replacement of the Pro(6) residue and the dicarboxylic acid linker with 2,3-pyrazine-dicarboxylic acid furnished a highly selective nanomolar range hMC3R partial agonist (analogue 12, c[CO-2,3-pyrazine-CO-D-Phe-Arg-Trp-Lys]-NH2, EC50 = 27 nM, 70% max cAMP) and an hMC3R antagonist (analogue 13, c[CO-2,3-pyrazine-CO-D-Nal(2')-Arg-Trp-Lys]-NH2, IC50 = 23 nM). Modeling experiments suggest that 2,3-pyrazinedicarboxylic acid stabilizes a beta-turn-like structure with the D-Phe/D-Nal(2') residues, which explains the high potency of the corresponding peptides. Placement of a Nle residue in position 6 produced a hMC3R/hMC5R antagonist (analogue 15, c[CO-(CH 2)2-CO-Nle-D-Nal(2')-Arg-Trp-Lys]-NH2, IC50 = 12 and 17 nM, respectively), similarly to the previously described cyclic gamma-melanocyte-stimulating hormone (gamma-MSH)-derived hMC3R/hMC5R antagonists. These newly developed melanotropins will serve as critical biochemical tools for elucidating the full spectrum of functions performed by the physiologically important melanocortin-3 receptor.
Figures




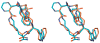



References
-
- Hadley ME, editor. The Melanotropic Peptides. I–III CRC Press; Boca Raton, FL: 1988.
-
- Cone RD. The Melanocortin System. Ann NY Acad Sci. 2003;994:1–387. - PubMed
-
- Cone RD, editor. The Melanocortin Receptors. Humana Press; Totowa, NJ: 2000.
-
- Vaudry H, Eberle AN. The Melanotropic Peptides. Ann NY Acad Sci. 1993;680:1–687.
-
- Gispen WH, Isaacson RL. ACTH-Induced Excessive Grooming in the Rat. Pharmacol Ther. 1981;12:209–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

