Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis
- PMID: 18088417
- PMCID: PMC2211489
- DOI: 10.1186/1471-213X-7-136
Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis
Abstract
Background: Germ cells arise from a small group of cells that express markers of pluripotency including OCT4. In humans formation of gonadal compartments (cords in testis, nests in ovary) takes place during the 1st trimester (6-8 weeks gestation). In the 2nd trimester germ cells can enter meiotic prophase in females whereas in males this does not occur until puberty. We have used qRTPCR, Westerns and immunohistochemical profiling to determine which of the germ cell subtypes in the human fetal gonads express OCT4, DAZL and VASA, as these have been shown to play an essential role in germ cell maturation in mice.
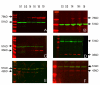
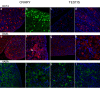
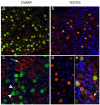
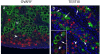
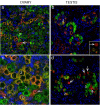
Results: OCT4 mRNA and protein were detected in extracts from both 1st and 2nd trimester ovaries and testes. In ovarian extracts a marked increase in expression of VASA and DAZL mRNA and protein occurred in the 2nd trimester. In testicular extracts VASA mRNA and protein were low/undetectable in 1st trimester and increased in the 2nd trimester whereas the total amount of DAZL did not seem to change. During the 1st trimester, germ cells were OCT4 positive but did not express VASA. These results are in contrast to the situation in mice where expression of Vasa is initiated in Oct4 positive primordial germ cells as they enter the gonadal ridge. In the 2nd trimester germ cells with intense cytoplasmic staining for VASA were present in both sexes; these cells were OCT4 negative. DAZL expression overlapped with both OCT4 and VASA and changed from the nuclear to the cytoplasmic compartment as cells became OCT4-negative. In males, OCT4-positive and VASA-positive subpopulations of germ cells coexisted within the same seminiferous cords but in the ovary there was a distinct spatial distribution of cells with OCT4 expressed by smaller, peripherally located, germ cells whereas DAZL and VASA were immunolocalised to larger (more mature) centrally located cells.
Conclusion: OCT4, DAZL and VASA are expressed by human fetal germ cells but their patterns of expression are temporally and spatially distinct. In the 1st trimester OCT4 was detected in most germ cells. In the 2nd trimester the onset of expression of VASA was associated with the formation of oocytes and spermatogonia both of which were OCT-4 negative. Relocation of DAZL from nucleus to cytoplasm paralleled the down regulation of OCT4 and the onset of expression of VASA. These data reveal similarities between the expression of key regulatory proteins within germ cells as they mature in male and female fetal human gonads suggesting that in the female these maturational changes are not determined by entry into meiosis.
Figures
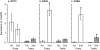
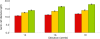
References
-
- Wartenberg H. Differentiation and development of the testes. In: Burger H, de Kretser DM, editor. The Testis. 2nd. New York , Raven Press; 1981. pp. 39–81. (Comprehensive Endocrinology). Martini L.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

