Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells
- PMID: 18089848
- PMCID: PMC2254535
- DOI: 10.1182/blood-2007-06-093237
Cross-talk between RhoH and Rac1 in regulation of actin cytoskeleton and chemotaxis of hematopoietic progenitor cells
Abstract
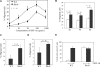
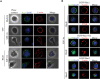
RhoH, a hematopoietic-specific and constitutively active member of the Rho guanosine triphosphatase (GTPase) family, has been implicated in the negative regulation of Rac GTPase-mediated signaling in hematopoietic cells. However, the molecular mechanisms underlying the functional interaction between RhoH and Rac in primary cells are poorly understood. Here we show that deletion of Rhoh in hematopoietic progenitor cells (HPCs) leads to increased stromal-derived factor-1alpha (SDF-1alpha)-induced chemotaxis and chemokinesis (random migration). The abnormally enhanced migration of Rhoh(-/-) HPCs is associated with increased Rac1 activity and translocation of Rac1 protein to the cell membrane, where it colocalizes with cortical filamentous-actin (F-actin) and lipid rafts. Expression of the dominant-negative mutant Rac1N17 inhibits the cortical F-actin assembly and chemotaxis of wild-type and Rhoh(-/-) HPCs to the same extent. Conversely, overexpression of RhoH in HPCs blocks the membrane translocation of Rac1-enhanced green fluorescence protein (EGFP) and active Rac1V12-EGFP proteins and impairs cortical F-actin assembly and chemotaxis in response to SDF-1alpha stimulation. Furthermore, we demonstrate that the subcellular localization and inhibitory function of RhoH in HPCs are regulated by C-terminal motifs, including a CKIF prenylation site. Together, we have identified an antagonistic role of RhoH in regulation of cortical F-actin assembly and chemotaxis via suppressing Rac1 membrane targeting and activation in primary HPCs.
Figures




References
-
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. - PubMed
-
- Symons M, Settleman J. Rho family GTPases: more than simple switches. Trends Cell Biol. 2000;10:415–419. - PubMed
-
- Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–732. - PubMed
-
- Gu Y, Filippi MD, Cancelas JA, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

