Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events
- PMID: 18090393
- PMCID: PMC3724474
- DOI: 10.1097/QAD.0b013e3282f2306e
Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events
Abstract
Objective: To describe the safety and tolerability of zidovudine, lamivudine, and efavirenz in a low-income setting.
Design: We conducted a prospective cohort study in a workplace HAART programme in South Africa, which uses a first-line regimen of efavirenz, zidovudine, and lamivudine and provides routine clinical and laboratory monitoring 6-monthly pre-HAART and at 2, 6, 12, 24, 36, 48 weeks during HAART.
Methods: We assessed the incidence of specified clinical and laboratory events (AIDS Clinical Trials Group grade 3 or higher) and associated regimen changes, hospitalizations, and deaths one year before HAART initiation and one year on-HAART using person-year analysis.
Results: Between November 2002 and October 2005, 853 subjects (98% male, median age 40 years, and median CD4 cell count at HAART initiation 186 cells/mul) met enrollment criteria. The incidence of events on-HAART was higher than pre-HAART for neutropenia and nausea/vomiting. Dizziness was common early after HAART initiation (not evaluated pre-HAART). Of those with neutropenia, 88% had no apparent clinical consequences. The incidence of anemia, hepatotoxicity, peripheral neuropathy, and rash was similar or higher pre-HAART than on-HAART. Mean hemoglobin rose during the time on-HAART and was higher at 24 and 48 weeks than at baseline (P < 0.001).
Discussion: This regimen was well tolerated with a short-term increase in neutropenia, nausea, and probably neurocerebellar events. Most significantly, in contrast to reports from high-income countries, we observed a long-term improvement in the hemoglobin concentration.
Figures
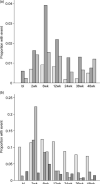
 Anemia;
Anemia;  neutropenia;
neutropenia;  hepatotoxicity. (b) Clinical events.
hepatotoxicity. (b) Clinical events.  Rash;
Rash;  nausea/vomiting;
nausea/vomiting;  neurocerebellar;
neurocerebellar;  neuropathy. Number of subjects evaluated during each time interval: baseline, 853; 2 weeks, 628; 6 weeks, 705; 12 weeks, 661; 24 weeks, 589; 36 weeks, 507; 48 weeks, 451. No baseline neurocerebellar data were available. bl, Baseline; wk, week after HAART initiation.
neuropathy. Number of subjects evaluated during each time interval: baseline, 853; 2 weeks, 628; 6 weeks, 705; 12 weeks, 661; 24 weeks, 589; 36 weeks, 507; 48 weeks, 451. No baseline neurocerebellar data were available. bl, Baseline; wk, week after HAART initiation.
Similar articles
-
Efficacy and tolerability of long-term efavirenz plus nucleoside reverse transcriptase inhibitors for HIV-1 infection.AIDS. 2008 Jan 11;22(2):275-9. doi: 10.1097/QAD.0b013e3282f21b9d. AIDS. 2008. PMID: 18097230 Clinical Trial.
-
Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides. Dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384.J Acquir Immune Defic Syndr. 2007 Aug 15;45(5):508-14. doi: 10.1097/QAI.0b013e3181142d26. J Acquir Immune Defic Syndr. 2007. PMID: 17589373 Clinical Trial.
-
Long-term safety and tolerability of the lamivudine/abacavir combination as components of highly active antiretroviral therapy.Drug Saf. 2006;29(9):811-26. doi: 10.2165/00002018-200629090-00005. Drug Saf. 2006. PMID: 16944966
-
Effects of Highly Active Antiretroviral Therapy on Albuminuria in HIVinfected Persons.Infect Disord Drug Targets. 2020;20(3):374-384. doi: 10.2174/1871526519666190807155025. Infect Disord Drug Targets. 2020. PMID: 31389318
-
Quadruple nucleoside therapy with zidovudine, lamivudine, abacavir and tenofovir in the treatment of HIV.Antivir Ther. 2007;12(5):695-703. Antivir Ther. 2007. PMID: 17713153 Review.
Cited by
-
Long-term virological, immunological and mortality outcomes in a cohort of HIV-infected female sex workers treated with highly active antiretroviral therapy in Africa.BMC Public Health. 2011 Sep 14;11:700. doi: 10.1186/1471-2458-11-700. BMC Public Health. 2011. PMID: 21917177 Free PMC article.
-
Evaluation of an immunoassay for determination of plasma efavirenz concentrations in resource-limited settings.J Int AIDS Soc. 2014 Jun 5;17(1):18979. doi: 10.7448/IAS.17.1.18979. eCollection 2014. J Int AIDS Soc. 2014. PMID: 24909561 Free PMC article.
-
Brief Report: Late Efavirenz-Induced Ataxia and Encephalopathy: A Case Series.J Acquir Immune Defic Syndr. 2017 Aug 15;75(5):577-579. doi: 10.1097/QAI.0000000000001451. J Acquir Immune Defic Syndr. 2017. PMID: 28520619 Free PMC article.
-
Incidence of Severe Neutropenia in HIV-Infected People Starting Antiretroviral Therapy in West Africa.PLoS One. 2017 Jan 25;12(1):e0170753. doi: 10.1371/journal.pone.0170753. eCollection 2017. PLoS One. 2017. PMID: 28122041 Free PMC article.
-
Five-year outcomes of initial patients treated in Botswana's National Antiretroviral Treatment Program.AIDS. 2008 Nov 12;22(17):2303-11. doi: 10.1097/QAD.0b013e3283129db0. AIDS. 2008. PMID: 18981769 Free PMC article.
References
-
- Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach, 3 by 5. 1st ed. World Health Organization; Geneva, Switzerland: 2002.
-
- Coghlan ME, Sommadossi JP, Jhala NC, Many WJ, Saag MS, Johnson VA. Symptomatic lactic acidosis in hospitalized anti-retroviral-treated patients with human immunodeficiency virus infection: a report of 12 cases. Clin Infect Dis. 2001;33:1914–1921. - PubMed
-
- Anekthananon T, Ratanasuwan W, Techasathit W, Suwanagool S, Auwanit W. 76 Weeks study of safety and efficacy of a simplified fixed-dose combination of stavudine, lamivudine, and nevirapine for the treatment of advanced HIV-infected patients.. 3rd IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro, Brazil. 24–27 July 2006; Abstract TuPe11.8C08.
-
- Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. - PubMed
-
- Geddes R, Knight S, Moosa MY, Reddi A, Uebel K, Sunpath H. A high incidence of nucleoside reverse transcriptase inhibitor (NRTI)-induced lactic acidosis in HIV-infected patients in a South African context. S Afr Med J. 2006;96:722–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

