Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity
- PMID: 18090922
- PMCID: PMC3034246
- DOI: 10.1097/nen.0b013e31815c5efb
Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity
Abstract
There is uncertainty regarding the association of cognitive decline in Alzheimer disease (AD) with classic histopathologic features- neurofibrillary tangles (NFTs) and "neuritic" amyloid plaques (NPs). This uncertainty fuels doubts about the diagnostic importance of NFTs and NPs and leads to confusion regarding hypotheses of AD pathogenesis. Three hundred ninety subjects who underwent longitudinal premortem clinical workup and postmortem quantitative neuropathologic assessment served as the group to address this issue. Subjects with concomitant brain disease(s) were analyzed independently to more accurately assess the contribution of distinct pathologies to cognitive decline. More than 60% of patients of all age groups had important non-AD brain pathologies. However, subjects without superimposed brain diseases showed strong correlations between AD-type pathology counts (NFTs > NPs) and premortem Mini-Mental State Examination scores. The observed correlation was stronger in isocortex than in allocortex and was maintained across age groups including patients older than 90 years. A theoretical model is proposed in which our results are interpreted to support the "amyloid cascade hypothesis" of AD pathogenesis. Our data show that there are many important contributory causes to cognitive decline in older persons. However, NFTs and NPs should not be dismissed as irrelevant in AD based on clinicopathologic correlation.
Figures


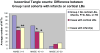


References
-
- Alzheimer A. Uber egenartige Krankheitsfalle des spateren Alters. Z Ges Neurol. 1911;4:356–85.
-
- Lee HG, Zhu X, Castellani RJ, et al. Amyloid-β in Alzheimer disease: The null versus the alternate hypotheses. J Pharmacol Exp Ther. 2007;321:823–29. - PubMed
-
- Wirths O, Multhaup G, Bayer TA. A modified β-amyloid hypothesis: Intraneuronal accumulation of the β-amyloid peptide—the first step of a fatal cascade. J Neurochem. 2004;91:513–20. - PubMed
-
- Lesne S, Koh MT, Kotilinek L, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

