What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain
- PMID: 18091986
- PMCID: PMC2144768
- DOI: 10.1371/journal.pone.0001292
What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain
Abstract
Background: Recent neuroscientific evidence suggests that empathy for pain activates similar neural representations as the first-hand experience of pain. However, empathy is not an all-or-none phenomenon but it is strongly malleable by interpersonal, intrapersonal and situational factors. This study investigated how two different top-down mechanisms - attention and cognitive appraisal - affect the perception of pain in others and its neural underpinnings.
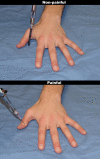
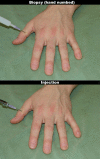
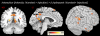
Methodology/principal findings: We performed one behavioral (N = 23) and two functional magnetic resonance imaging (fMRI) experiments (N = 18). In the first fMRI experiment, participants watched photographs displaying painful needle injections, and were asked to evaluate either the sensory or the affective consequences of these injections. The role of cognitive appraisal was examined in a second fMRI experiment in which participants watched injections that only appeared to be painful as they were performed on an anesthetized hand. Perceiving pain in others activated the affective-motivational and sensory-discriminative aspects of the pain matrix. Activity in the somatosensory areas was specifically enhanced when participants evaluated the sensory consequences of pain. Perceiving non-painful injections into the anesthetized hand also led to signal increase in large parts of the pain matrix, suggesting an automatic affective response to the putatively harmful stimulus. This automatic response was modulated by areas involved in self/other distinction and valence attribution - including the temporo-parietal junction and medial orbitofrontal cortex.
Conclusions/significance: Our findings elucidate how top-down control mechanisms and automatic bottom-up processes interact to generate and modulate other-oriented responses. They stress the role of cognitive processing in empathy, and shed light on how emotional and bodily awareness enable us to evaluate the sensory and affective states of others.
Conflict of interest statement
Figures




References
-
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, et al. Viewing facial expression of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. - PubMed
-
- Cheng Y, Lin C-P, Yang C-Y, Wang C-C, Hung D, et al. 2007. The perception of pain in others modulates somatosensory oscillations. Poster presented at the 13th Annual Meeting of the Organization for Human Brain Mapping, Chicago, IL, June 10-14, 2007.
-
- Jackson P, Brunet E, Meltzoff A, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. - PubMed
-
- Jackson P, Meltzoff A, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. - PubMed
-
- Lamm C, Batson C, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

