SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana
- PMID: 18091987
- PMCID: PMC2129116
- DOI: 10.1371/journal.pone.0001312
SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana
Abstract
Objective: The objective of this trial was to determine the effectiveness of 1.0% C31G (SAVVY) in preventing male-to-female vaginal transmission of HIV infection among women at high risk.
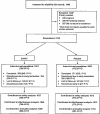
Methodology/principal findings: This was a Phase 3, double-blind, randomized, placebo-controlled trial. Participants made up to 12 monthly visits for HIV testing, adverse event reporting, and study product supply. The study was conducted between March 2004 and February 2006 in Accra and Kumasi, Ghana. We enrolled 2142 HIV-negative women at high risk of HIV infection, and randomized them to SAVVY or placebo gel. Main outcome measures were the incidence of HIV-1 and HIV-2 infection as determined by detection of HIV antibodies from oral mucosal transudate specimens and adverse events. We accrued 790 person-years of follow-up in the SAVVY group and 772 person-years in the placebo group. No clinically significant differences in the overall frequency of adverse events, abnormal pelvic examination findings, or abnormal laboratory results were seen between treatment groups. However, more participants in the SAVVY group reported reproductive tract adverse events than in the placebo group (13.0% versus 9.4%). Seventeen HIV seroconversions occurred; eight in participants randomized to SAVVY and nine in participants receiving placebo. The Kaplan-Meier estimates of the cumulative probability of HIV infection through 12 months were 0.010 in the SAVVY group and 0.011 in the placebo group (p = 0.731), with a hazard ratio (SAVVY versus placebo) of 0.88 (95% confidence interval 0.33, 2.27). Because of a lower-than-expected HIV incidence, we were unable to achieve the required number of HIV infections (66) to obtain the desired study power.
Conclusions/significance: SAVVY was not associated with increased adverse events overall, but was associated with higher reporting of reproductive adverse events. Our data are insufficient to conclude whether SAVVY is effective at preventing HIV infection relative to placebo.
Trial registration: ClinicalTrials.gov NCT00129532.
Conflict of interest statement
Figures
References
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–4. - PubMed
-
- Roddy RE, Cordero M, Cordero C, Fortney JA. A dosing study of nonoxynol-9 and genital irritation. Int J STD AIDS. 1993;4:165–70. - PubMed
-
- Niruthisard S, Roddy RE, Chutivongse S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18:176–9. - PubMed
-
- Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. - PubMed
-
- Biosyn, Inc. Investigator's Brochure: 1% C31G. 2005
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical