Neural induction from ES cells portrays default commitment but instructive maturation
- PMID: 18092007
- PMCID: PMC2121127
- DOI: 10.1371/journal.pone.0001349
Neural induction from ES cells portrays default commitment but instructive maturation
Abstract
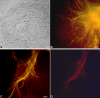
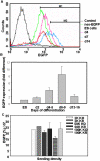
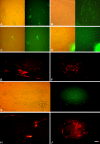
The neural induction has remained a debatable issue pertaining to whether it is a mere default process or it involves precise instructive cues. We have chosen the embryonic stem (ES) cell model to address this issue. In a devised monoculture strategy, the cell-cell interaction availed through optimum cell plating density could define the niche for the attainment of efficient in vitro neurogenesis from the ES cells. The medium plating density was found ideal in generating optimum number of progenitors and also yielded about 80% mature neurons in a serum free culture set up barring any exogenous inducers. We could also demarcate and quantify the neural stem cells/progenitors among the heterogeneous cell population of differentiating ES cells using nestin intron II driven EGFP expression as a tool. The one week post-plating was determined to be the critical time window for optimum neural progenitor generation from ES cells that helped us further in purifying these cells and in demonstrating their proliferation and multipotent differentiation potential. Seeding cells at varying densities, we could decipher an interesting paradoxical scenario that interlinked both commitment and maturation with the initial plating density having a vital influence on neuronal maturation but not specification and the secretory factors were apparently playing a key role during this process. Thus it was comprehended that, the neural specification was a default process independent of exogenous factors and cellular interaction. Conversely, a defined number of cells at the specification stage itself seemed critical to provide an auto-/paracrine means of signaling threshold for the maturation process to materialize.
Conflict of interest statement
Figures

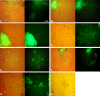
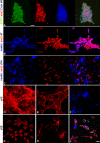
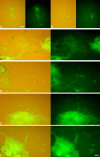
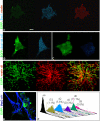


Similar articles
-
Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo.PLoS One. 2009 Jul 21;4(7):e6286. doi: 10.1371/journal.pone.0006286. PLoS One. 2009. PMID: 19621087 Free PMC article.
-
Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism.Neuron. 2001 Apr;30(1):65-78. doi: 10.1016/s0896-6273(01)00263-x. Neuron. 2001. PMID: 11343645
-
Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11796-801. doi: 10.1073/pnas.0803078105. Epub 2008 Aug 12. Proc Natl Acad Sci U S A. 2008. PMID: 18697938 Free PMC article.
-
Optimization and integration of expansion and neural commitment of mouse embryonic stem cells.Biotechnol Appl Biochem. 2008 Feb;49(Pt 2):105-12. doi: 10.1042/BA20070011. Biotechnol Appl Biochem. 2008. PMID: 17596123
-
Neural induction and neural stem cell development.Regen Med. 2006 Sep;1(5):635-52. doi: 10.2217/17460751.1.5.635. Regen Med. 2006. PMID: 17465732 Review.
Cited by
-
Notch exhibits ligand bias and maneuvers stage-specific steering of neural differentiation in embryonic stem cells.Mol Cell Biol. 2010 Apr;30(8):1946-57. doi: 10.1128/MCB.01419-09. Epub 2010 Feb 12. Mol Cell Biol. 2010. PMID: 20154142 Free PMC article.
-
The class I-specific HDAC inhibitor MS-275 modulates the differentiation potential of mouse embryonic stem cells.Biol Open. 2013 Aug 22;2(10):1070-7. doi: 10.1242/bio.20135587. eCollection 2013. Biol Open. 2013. PMID: 24167717 Free PMC article.
-
Deletion of the Dishevelled family of genes disrupts anterior-posterior axis specification and selectively prevents mesoderm differentiation.Dev Biol. 2020 Aug 15;464(2):161-175. doi: 10.1016/j.ydbio.2020.05.010. Epub 2020 Jun 21. Dev Biol. 2020. PMID: 32579954 Free PMC article.
-
Genetic control of the pluripotency epigenome determines differentiation bias in mouse embryonic stem cells.EMBO J. 2022 Dec 17;41(2):e109445. doi: 10.15252/embj.2021109445. Epub 2021 Dec 21. EMBO J. 2022. PMID: 34931323 Free PMC article.
-
Engraftment of mouse embryonic stem cells differentiated by default leads to neuroprotection, behaviour revival and astrogliosis in parkinsonian rats.PLoS One. 2013 Sep 12;8(9):e72501. doi: 10.1371/journal.pone.0072501. eCollection 2013. PLoS One. 2013. PMID: 24069147 Free PMC article.
References
-
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–17. - PubMed
-
- Munoz-Sanjuan I, Hemmati-Brivanlou A. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. - PubMed
-
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003a;21:183–186. - PubMed
-
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, et al. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

