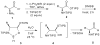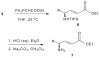Enantiomerically pure alpha-amino aldehydes from silylated alpha-amino acids
- PMID: 18092797
- PMCID: PMC2563420
- DOI: 10.1021/ol7028993
Enantiomerically pure alpha-amino aldehydes from silylated alpha-amino acids
Abstract
The disilylation of alpha-amino acids 1 to provide 2 (72-87%) was achieved without racemization. An unprecedented borane-mediated semi-reduction strategy was devised to convert 2 to stable, isolable oxazaborolidines 3 (100%) which were hydrolyzed to provide 5 (49-60%) as pure, stable compounds. Analysis of the Mosher amides (8) of the gamma-amino esters 7 reveals that < or =2% racemization occurs in the 1 --> 8 conversions.
Figures
References
-
- Jurczak J, Golebiowski A. Chem Rev. 1989;89:149. and ref. cited therein.
- Reetz MT. Chem Rev. 1999;99:1121. - PubMed
- Liang X, Andersch Bols M. J Chem Soc, Perkin Trans 1. 2001:2136.
-
- Kwon S, Myers AG. J Am Chem Soc. 2005;127:16796. - PubMed
- Nicolaou KC, Hummel CW, Pitsinos EN, Nakada M, Smith AL, Shibayama K, Saimoto H. J Am Chem Soc. 1992;114:10082.
- Corey EJ, Reichard GA. J Am Chem Soc. 1992;114:10677.
- Vedejs E, Naidu BN, Klapars A, Warner DL, Li V, Na Y, Kohn H. J Am Chem Soc. 2003;125:15796. - PubMed
- Myers AG, Kung DW. J Am Chem Soc. 1999;121:10828.
- Lin S, Yang Z, Kwok BHB, Koldobskiy M, Crews CM, Danishefsky SM. J Am Chem Soc. 2004;126:6347. - PMC - PubMed
- Boger DL, Colleti SL, Honda T, Menezes RF. J Am Chem Soc. 1994;116:5607.
- Hagihara M, Schreiber SL. J Am Chem Soc. 1992;114:6570.
-
- Garner P, Park JM. Org Synth. 1992;70:18.
- Roush WR, Hunt JA. J Org Chem. 1995;60:798.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources