Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model
- PMID: 18092886
- PMCID: PMC2140085
- DOI: 10.1371/journal.pmed.0040344
Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model
Abstract
Background: Availability of an ultra-short-course drug regimen capable of curing patients with tuberculosis in 2 to 3 mo would significantly improve global control efforts. Because immediate prospects for novel treatment-shortening drugs remain uncertain, we examined whether better use of existing drugs could shorten the duration of treatment. Rifapentine is a long-lived rifamycin derivative currently recommended only in once-weekly continuation-phase regimens. Moxifloxacin is an 8-methoxyfluoroquinolone currently used in second-line regimens.
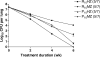
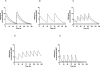
Methods and findings: Using a well-established mouse model with a high bacterial burden and human-equivalent drug dosing, we compared the efficacy of rifapentine- and moxifloxacin-containing regimens with that of the standard daily short-course regimen based on rifampin, isoniazid, and pyrazinamide. Bactericidal activity was assessed by lung colony-forming unit counts, and sterilizing activity was assessed by the proportion of mice with culture-positive relapse after 2, 3, 4, and 6 mo of treatment. Here, we demonstrate that replacing rifampin with rifapentine and isoniazid with moxifloxacin dramatically increased the activity of the standard daily regimen. After just 2 mo of treatment, mice receiving rifapentine- and moxifloxacin-containing regimens were found to have negative lung cultures, while those given the standard regimen still harbored 3.17 log10 colony-forming units in the lungs (p < 0.01). No relapse was observed after just 3 mo of treatment with daily and thrice-weekly administered rifapentine- and moxifloxacin-containing regimens, whereas the standard daily regimen required 6 mo to prevent relapse in all mice.
Conclusions: Rifapentine should no longer be viewed solely as a rifamycin for once-weekly administration. Our results suggest that treatment regimens based on daily and thrice-weekly administration of rifapentine and moxifloxacin may permit shortening the current 6 mo duration of treatment to 3 mo or less. Such regimens warrant urgent clinical investigation.
Conflict of interest statement
Figures


References
-
- Stop TB Partnership and World Health Organization. Global Plan to Stop TB 2006–2015. Geneva: World Health Organization; 2006. (WHO/HTM/STB/2006.35). Available: http://www.who.int/tb/publications/2006/en. Accessed 1 November 2007.
-
- O'Brien RJ, Nunn PP. The need for new drugs against tuberculosis. Obstacles, opportunities and next steps. Am J Respir Crit Care Med. 2001;163:1055–1058. - PubMed
-
- Spigelman M, Gillespie S. Tuberculosis drug development pipeline: progress and hope. Lancet. 2006;367:945–947. - PubMed
-
- Andries K, Verhasselt P, Guillemont J, Gohlman H, Neefs J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis . Science. 2005;307:223–227. - PubMed
-
- Glickman SW, Rasiel EB, Hamilton CD, Kubataev A, Schulman KA. A portfolio model of drug development for tuberculosis. Science. 2006;311:1246–1247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

