An atypical orthologue of 6-pyruvoyltetrahydropterin synthase can provide the missing link in the folate biosynthesis pathway of malaria parasites
- PMID: 18093090
- PMCID: PMC2229834
- DOI: 10.1111/j.1365-2958.2007.06073.x
An atypical orthologue of 6-pyruvoyltetrahydropterin synthase can provide the missing link in the folate biosynthesis pathway of malaria parasites
Abstract
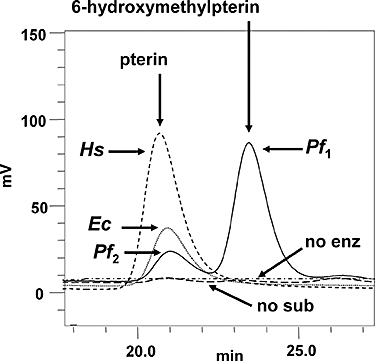
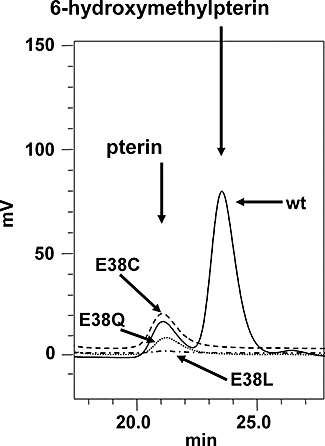
Folate metabolism in malaria parasites is a long-standing, clinical target for chemotherapy and prophylaxis. However, despite determination of the complete genome sequence of the lethal species Plasmodium falciparum, the pathway of de novo folate biosynthesis remains incomplete, as no candidate gene for dihydroneopterin aldolase (DHNA) could be identified. This enzyme catalyses the third step in the well-characterized pathway of plants, bacteria, and those eukaryotic microorganisms capable of synthesizing their own folate. Utilizing bioinformatics searches based on both primary and higher protein structures, together with biochemical assays, we demonstrate that P. falciparum cell extracts lack detectable DHNA activity, but that the parasite possesses an unusual orthologue of 6-pyruvoyltetrahydropterin synthase (PTPS), which simultaneously gives rise to two products in comparable amounts, the predominant of which is 6-hydroxymethyl-7,8-dihydropterin, the substrate for the fourth step in folate biosynthesis (catalysed by 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase; PPPK). This can provide a bypass for the missing DHNA activity and thus a means of completing the biosynthetic pathway from GTP to dihydrofolate. Supported by site-directed mutagenesis experiments, we ascribe the novel catalytic activity of the malarial PTPS to a Cys to Glu change at its active site relative to all previously characterized PTPS molecules, including that of the human host.
Figures


Similar articles
-
Plasmodium falciparum: a paradigm for alternative folate biosynthesis in diverse microorganisms?Trends Parasitol. 2008 Nov;24(11):502-8. doi: 10.1016/j.pt.2008.08.004. Epub 2008 Sep 19. Trends Parasitol. 2008. PMID: 18805734 Free PMC article.
-
6-pyruvoyltetrahydropterin synthase paralogs replace the folate synthesis enzyme dihydroneopterin aldolase in diverse bacteria.J Bacteriol. 2009 Jul;191(13):4158-65. doi: 10.1128/JB.00416-09. Epub 2009 Apr 24. J Bacteriol. 2009. PMID: 19395485 Free PMC article.
-
Structural basis of a novel activity of bacterial 6-pyruvoyltetrahydropterin synthase homologues distinct from mammalian 6-pyruvoyltetrahydropterin synthase activity.Acta Crystallogr D Biol Crystallogr. 2014 May;70(Pt 5):1212-23. doi: 10.1107/S1399004714002016. Epub 2014 Apr 26. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 24816091
-
The folate metabolic network of Falciparum malaria.Mol Biochem Parasitol. 2013 Mar;188(1):51-62. doi: 10.1016/j.molbiopara.2013.02.003. Epub 2013 Feb 27. Mol Biochem Parasitol. 2013. PMID: 23454873 Review.
-
Novel mutation affecting the pterin-binding site of PTS gene and review of PTS mutations in Thai patients with 6-pyruvoyltetrahydropterin synthase deficiency.J Inherit Metab Dis. 2009 Dec;32 Suppl 1:S279-82. doi: 10.1007/s10545-009-1221-x. Epub 2009 Oct 13. J Inherit Metab Dis. 2009. PMID: 19830588 Review.
Cited by
-
Organic cofactors in the metabolism of Dehalococcoides mccartyi strains.Philos Trans R Soc Lond B Biol Sci. 2013 Mar 11;368(1616):20120321. doi: 10.1098/rstb.2012.0321. Print 2013 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23479751 Free PMC article.
-
Plasmodium falciparum: a paradigm for alternative folate biosynthesis in diverse microorganisms?Trends Parasitol. 2008 Nov;24(11):502-8. doi: 10.1016/j.pt.2008.08.004. Epub 2008 Sep 19. Trends Parasitol. 2008. PMID: 18805734 Free PMC article.
-
The Rickettsia Endosymbiont of Ixodes pacificus Contains All the Genes of De Novo Folate Biosynthesis.PLoS One. 2015 Dec 9;10(12):e0144552. doi: 10.1371/journal.pone.0144552. eCollection 2015. PLoS One. 2015. PMID: 26650541 Free PMC article.
-
A unique dual activity amino acid hydroxylase in Toxoplasma gondii.PLoS One. 2009;4(3):e4801. doi: 10.1371/journal.pone.0004801. Epub 2009 Mar 11. PLoS One. 2009. PMID: 19277211 Free PMC article.
-
CRISPR-based functional profiling of the Toxoplasma gondii genome during acute murine infection.Nat Microbiol. 2024 Sep;9(9):2323-2343. doi: 10.1038/s41564-024-01754-2. Epub 2024 Jul 8. Nat Microbiol. 2024. PMID: 38977907 Free PMC article.
References
-
- Aspinall TV, Joynson DHM, Guy E, Hyde JE, Sims PFG. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J Inf Dis. 2002;185:1637–1643. - PubMed
-
- Bermingham A, Derrick JP. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. Bioessays. 2002;24:637–648. - PubMed
-
- Bracher A, Eisenreich W, Schramek N, Ritz H, Gotze E, Herrmann A, et al. Biosynthesis of pteridines – NMR studies on the reaction mechanisms of GTP cyclohydrolase I, pyruvoyltetrahydropterin synthase, and sepiapterin reductase. J Biol Chem. 1998;273:28132–28141. - PubMed
-
- Brooks DR, Wang P, Read M, Watkins WM, Sims PFG, Hyde JE. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase – dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur J Biochem. 1994;224:397–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

