Retinoic acid is a potential dorsalising signal in the late embryonic chick hindbrain
- PMID: 18093305
- PMCID: PMC2266733
- DOI: 10.1186/1471-213X-7-138
Retinoic acid is a potential dorsalising signal in the late embryonic chick hindbrain
Abstract
Background: Human retinoic acid teratogenesis results in malformations of dorsally derived hindbrain structures such as the cerebellum, noradrenergic hindbrain neurons and the precerebellar system. These structures originate from the rhombic lip and adjacent dorsal precursor pools that border the fourth ventricle roofplate. While retinoic acid synthesis is known to occur in the meninges that blanket the hindbrain, the particular sensitivity of only dorsal structures to disruptions in retinoid signalling is puzzling. We therefore looked for evidence within the neural tube for more spatiotemporally specific signalling pathways using an in situ hybridisation screen of known retinoic acid pathway transcripts.
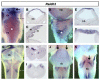
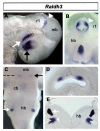
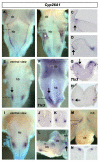
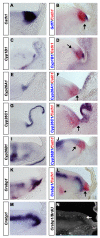
Results: We find that there are highly restricted domains of retinoic acid synthesis and breakdown within specific hindbrain nuclei as well as the ventricular layer and roofplate. Intriguingly, transcripts of cellular retinoic acid binding protein 1 are always found at the interface between dividing and post-mitotic cells. By contrast to earlier stages of development, domains of synthesis and breakdown in post-mitotic neurons are co-localised. At the rhombic lip, expression of the mRNA for retinoic acid synthesising and catabolising enzymes is spatially highly organised with respect to the Cath1-positive precursors of migratory precerebellar neurons.
Conclusion: The late developing hindbrain shows patterns of retinoic acid synthesis and use that are distinct from the well characterised phase of rostrocaudal patterning. Selected post-mitotic populations, such as the locus coeruleus, appear to both make and break down retinoic acid suggesting that a requirement for an autocrine, or at least a highly localised paracrine signalling network, might explain its acute sensitivity to retinoic acid disruption. At the rhombic lip, retinoic acid is likely to act as a dorsalising factor in parallel with other roofplate signalling pathways. While its precise role is unclear, retinoic acid is potentially well placed to regulate temporally determined cell fate decisions within the rhombic lip precursor pool.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

