Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners
- PMID: 18093311
- PMCID: PMC2233628
- DOI: 10.1186/1471-2180-7-115
Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners
Abstract
Background: Most studies of the vaginal microflora have been based on culture or on qualitative molecular techniques. Here we applied existing real-time PCR formats for Lactobacillus crispatus, L. gasseri and Gardnerella vaginalis and developed new formats for Atopobium vaginae, L. iners and L. jensenii to obtain a quantitative non culture-based determination of these species in 71 vaginal samples from 32 pregnant and 28 non-pregnant women aged between 18 and 45 years.
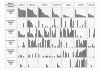
Results: The 71 vaginal microflora samples of these women were categorized, using the Ison and Hay criteria, as refined by Verhelst et al. (2005), as follows: grade Ia: 8 samples, grade Iab: 10, grade Ib: 13, grade I-like: 10, grade II: 11, grade III: 12 and grade IV: 7.L. crispatus was found in all but 5 samples and was the most frequent Lactobacillus species detected. A significantly lower concentration of L. crispatus was found in grades II (p < 0.0001) and III (p = 0.002) compared to grade I. L. jensenii was found in all grades but showed higher concentration in grade Iab than in grade Ia (p = 0.024). A. vaginae and G. vaginalis were present in high concentrations in grade III, with log10 median concentrations (log10 MC), respectively of 9.0 and 9.2 cells/ml. Twenty (38.5%) of the 52 G. vaginalis positive samples were also positive for A. vaginae. In grade II we found almost no L. iners (log10 MC: 0/ml) but a high concentration of L. gasseri (log10 MC: 8.7/ml). By contrast, in grade III we found a high concentration of L. iners (log10 MC: 8.3/ml) and a low concentration of L. gasseri (log10 MC: 0/ml). These results show a negative association between L. gasseri and L. iners (r = -0.397, p = 0.001) and between L. gasseri and A. vaginae (r = -0.408, p < 0.0001).
Conclusion: In our study we found a clear negative association between L. iners and L. gasseri and between A. vaginae and L. gasseri. Our results do not provide support for the generally held proposition that grade II is an intermediate stage between grades I and III, because L. gasseri, abundant in grade II is not predominant in grade III, whereas L. iners, abundant in grade III is present only in low numbers in grade II samples.
Figures
References
-
- Watts DH, Krohn MA, Hillier SL, Eschenbach DA. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990;75:52–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

