Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas
- PMID: 18093335
- PMCID: PMC2254376
- DOI: 10.1186/1479-5876-5-67
Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas
Abstract
Background: The prognosis for malignant gliomas remains dismal. We addressed the safety, feasibility and preliminary clinical activity of the vaccinations using autologous glioma cells and interleukin (IL)-4 gene transfected fibroblasts.
Methods: In University of Pittsburgh Cancer Institute (UPCI) protocol 95-033, adult participants with recurrent glioblastoma multiforme (GBM) or anaplastic astrocytoma (AA) received gross total resection (GTR) of the recurrent tumors, followed by two vaccinations with autologous fibroblasts retrovirally transfected with TFG-IL4-Neo-TK vector admixed with irradiated autologous glioma cells. In UPCI 99-111, adult participants with newly diagnosed GBM or AA, following GTR and radiation therapy, received two intradermal vaccinations with the TFG-IL4-Neo-TK-transfected fibroblasts admixed with type-1 dendritic cells (DC) loaded with autologous tumor lysate. The participants were evaluated for occurrence of adverse events, immune response, and clinical response by radiological imaging.
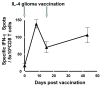
Results and discussion: In UPCI 95-033, only 2 of 6 participants received the vaccinations. Four other participants were withdrawn from the trial because of tumor progression prior to production of the cellular vaccine. However, both participants who received two vaccinations demonstrated encouraging immunological and clinical responses. Biopsies from the local vaccine sites from one participant displayed IL-4 dose-dependent infiltration of CD4+ as well as CD8+ T cells. Interferon (IFN)-gamma Enzyme-Linked Immuno-SPOT (ELISPOT) assay in another human leukocyte antigen (HLA)-A2+ participant demonstrated systemic T-cell responses against an HLA-A2-restricted glioma-associated antigen (GAA) epitope EphA2883-891. Moreover, both participants demonstrated clinical and radiological improvement with no evidence of allergic encephalitis, although both participants eventually succumbed with the tumor recurrence. In 99-111, 5 of 6 enrolled participants received scheduled vaccinations with no incidence of major adverse events. Monocyte-derived DCs produced high levels of IL-12 p70. Treatment was well tolerated; however, we were unable to observe detectable IFN-gamma post-vaccine responses or prolonged progression-free survival in these participants.
Conclusion: Feasibility challenges inherent in the generation of a patient-specific gene transfection-based vaccine strongly suggests the need for more practical formulations that would allow for the timely administration of vaccines. Nevertheless, successful generation of type-1 DCs and preliminary safety in the current study provide a strong rationale for further efforts to develop novel glioma vaccines.
Figures


References
-
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. - DOI - PMC - PubMed
-
- Dranoff G. The use of gene transfer in cancer immunotherapy. [Review] [48 refs] Forum. 1998;8:357–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

