Spontaneous immune responses against glioma-associated antigens in a long term survivor with malignant glioma
- PMID: 18093336
- PMCID: PMC2244605
- DOI: 10.1186/1479-5876-5-68
Spontaneous immune responses against glioma-associated antigens in a long term survivor with malignant glioma
Abstract
Background: In patients with high grade glioma, little is known regarding existence of naturally occurring adaptive T cell reactivity against glioma-associated antigens (GAAs). In this report, we characterized GAA-specific CD8+ T cells and innate immune cells in a patient who has survived with anaplastic astrocytoma (AA) for over 12 years without recurrence.
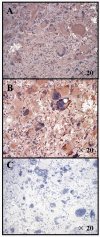
Methods: Peripheral blood mononuclear cells (PBMCs) derived from the long term survivor with AA were evaluated for the frequency, cytotoxic T lymphocyte (CTL) activity and differentiation status of CD8+ cells recognizing GAA-derived epitopes as well as relative numbers of other immune cell subsets. This patient's AA tissue was evaluated for expression of two GAAs EphA2 and interleukin-13 receptor alpha2 subunit (IL-13Ralpha2) by immunohistochemistry.
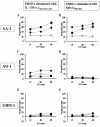
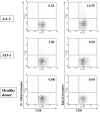
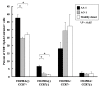
Results: The patient's tumor expressed both EphA2 and IL-13Ralpha2, and in vitro stimulated PBMC demonstrated superior EphA2883-891 and IL-13Ralpha2345-353-specific CTL reactivity compared to PBMC samples from two other patients with progressing malignant glioma. Unstimulated EphA2883-891-reactive CD8+ T cells contained high numbers of CD45RA-/CCR7- late effector and CD45RA-/CCR7+ central memory cells. Among other leukocyte subsets, elevated numbers of NK-T cells were found.
Conclusion: To our knowledge, the current study is one of the first demonstrating the presence of antigen-experienced, GAA-reactive CD8+ T cells in a patient who has survived with AA for over 12 years without recurrence. Further studies are warranted to determine whether the status of GAA-reactive CD8+ T cells dictates survival of patients and/or response to therapeutic vaccines.
Figures
References
-
- The Central Brain Tumor Registry of the United States Statistical Report: Primary Brain Tumors in the United States,1998-2002. Anonymous. 2005.
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–25; discussion 226-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

