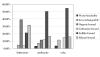Sequential solvent extraction for the modes of occurrence of selenium in coals of different ranks from the Huaibei Coalfield, China
- PMID: 18093341
- PMCID: PMC2242793
- DOI: 10.1186/1467-4866-8-14
Sequential solvent extraction for the modes of occurrence of selenium in coals of different ranks from the Huaibei Coalfield, China
Abstract
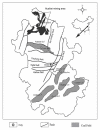
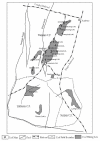
Forms of selenium in bituminous coal, anthracite, and cokeite (natural coke) from Huaibei Coalfield, Anhui, China, have been determined by sequential solvent extraction. The selenium content in bulk samples is 4.0, 2.4, and 2.0 microg/g in bituminous coal, anthracite, and cokeite, respectively. The six forms of selenium determined by six-step solvent extraction are water-leachable, ion-exchangeable, organic matter-associated, carbonate-associated, silicate-associated, and sulfide-associated. The predominant forms of selenium in bituminous coal are organic matter-associated (39.0%), sulfide-associated (21.1%), and silicate bound (31.8%); these three forms account for 92% of the total. The organic matter bound-selenium decrease dramatically from bituminous coal (39.0%) to anthracite (11.6%) and to cokeite (0%), indicating that organic matter bound selenium is converted to other forms during metamorphism of the coal, most likely sulfide-form. The sulfide-associated form increased remarkably from bituminous coal (21.1%) to anthracite (50.4%) and cokeite (54.5%), indicating the formation of selenium sulfide, possibly in pyrite during the transformation of bituminous coal to anthracite and cokeite. The silicate-associated selenium in bituminous coal (31.8%) is much higher than that in anthracite (16.4%) and cokeite (15.8%), indicating that silicate-associated selenium is partly converted to sulfide during metamorphism.
Figures
References
-
- Chen BH, Hong CJ, Kan HD. Exposures and health outcomes from outdoor air pollutants in China. Toxicology. 2004;198:291–300. - PubMed
-
- Florig HK. China's air pollution risks. Environmental Science Technology. 1997;31:274A–279A. - PubMed
-
- Ni B. New progress in high-precision and high resolution seismic exploration technology in coal industry of China. Acta Geological Sinica. 2000;74:311.
-
- Xu X, Chen C, Qi H. Development of coal combustion pollution control for SO2 and NOx in China. Fuel Processing Technology. 2000;62:153–160.
-
- Zhong Tang, Yang Wang. Efficient and environment friendly use of coal. Fuel Processing Technology. 2000;62:137–141.
LinkOut - more resources
Full Text Sources