Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids
- PMID: 18093525
- PMCID: PMC2770950
- DOI: 10.1016/j.neuron.2007.11.014
Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids
Abstract
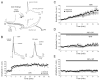
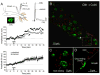
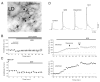
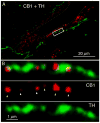
Endocannabinoids are well established as inhibitors of chemical synaptic transmission via presynaptic activation of the cannabinoid type 1 receptor (CB1R). Contrasting this notion, we show that dendritic release of endocannabinoids mediates potentiation of synaptic transmission at mixed (electrical and chemical) synaptic contacts on the goldfish Mauthner cell. Remarkably, the observed enhancement was not restricted to the glutamatergic component of the synaptic response but also included a parallel increase in electrical transmission. This effect involved the activation of CB1 receptors and was indirectly mediated via the release of dopamine from nearby varicosities, which in turn led to potentiation of the synaptic response via a cAMP-dependent protein kinase-mediated postsynaptic mechanism. Thus, endocannabinoid release can potentiate synaptic transmission, and its functional roles include the regulation of gap junction-mediated electrical synapses. Similar interactions between endocannabinoid and dopaminergic systems may be widespread and potentially relevant for the motor and rewarding effects of cannabis derivatives.
Figures




Comment in
-
Endocannabinoids mediate synaptic plasticity at mixed synapses.Neuron. 2007 Dec 20;56(6):945-6. doi: 10.1016/j.neuron.2007.12.005. Neuron. 2007. PMID: 18093517
References
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
-
- Bash R, Rubovitch V, Gafni M, Sarne Y. The stimulatory effect of cannabinoids on calcium uptake is mediated by Gs GTP-binding proteins and cAMP formation. Neurosignals. 2003;12:39–44. - PubMed
-
- Bonhaus DW, Chang LK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287:884–888. - PubMed
-
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

