Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes
- PMID: 18093644
- PMCID: PMC2692518
- DOI: 10.1016/j.biomaterials.2007.11.022
Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes
Abstract
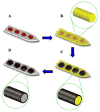
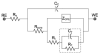
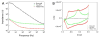
Neural prostheses transduce bioelectric signals to electronic signals at the interface between neural tissue and neural microelectrodes. A low impedance electrode-tissue interface is important for the quality of signal during recording as well as quantity of applied charge density during stimulation. However, neural microelectrode sites exhibit high impedance because of their small geometric surface area. Here we analyze nanostructured-conducting polymers that can be used to significantly decrease the impedance of microelectrode typically by about two orders of magnitude and increase the charge transfer capacity of microelectrodes by three orders of magnitude. In this study poly(pyrrole) (PPy) and poly(3,4-ethylenedioxythiophene) (PEDOT) nanotubes were electrochemically polymerized on the surface of neural microelectrode sites (1250 microm(2)). An equivalent circuit model comprising a coating capacitance in parallel with a pore resistance and interface impedance in series was developed and fitted to experimental results to characterize the physical and electrical properties of the interface. To confirm that the fitting parameters correlate with physical quantities of interface, theoretical equations were used to calculate the parameter values thereby validating the proposed model. Finally, an apparent diffusion coefficient was calculated for PPy film (29.2+/-1.1 x 10(-6) cm(2)/s), PPy nanotubes (PPy NTs) (72.4+/-3.3 x 10(-6) cm(2)/s), PEDOT film (7.4+/-2.1 x 10(-6) cm(2)/s), and PEDOT nanotubes (PEDOT NTs) (13.0+/-1.8 x 10(-6) cm(2)/s). The apparent diffusion coefficient of conducting polymer nanotubes was larger than the corresponding conducting polymer films.
Figures





References
-
- Gross GW, Rhoades BK, Reust DL, Schwalm FU. Stimulation of monolayer networks in culture through thin-film indium-tin oxide recording electrodes. J Neurosci Methods. 1993;50(2):131–143. - PubMed
-
- Thiebaud P, Beuret C, Koudelka-Hep M, Bove M, Martinoia S, Grattarola M, et al. An array of pt-tip microelectrodes for extracellular monitoring of activity of brain slices. Biosens Bioelectron. 1999;14(1):61–65. - PubMed
-
- Kovacs GTA. Introduction to the theory, design, and modeling of thin-film microelectrodes for neural interfaces. In: Stenger DA, McKenna TM, editors. Enabling technologies for cultured neural networks. London, U.K: Academic Press; 1994. pp. 121–165.
-
- Humayun MS, de Juan E, Weiland JD, Dagnelie G, Katona S, Greenberg R, et al. Pattern electrical stimulation of the human retina. Vision Research. 1999;39(15):2569–2576. - PubMed
-
- Rizzo JF, Wyatt J, Loewenstein J, Kelly S, Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Investigative Ophthalmology & Visual Science. 2003;44(12):5355–5361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

