Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3
- PMID: 18093802
- PMCID: PMC2233603
- DOI: 10.1016/j.cellsig.2007.11.005
Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3
Abstract
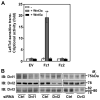
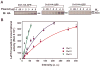
In the Drosophila, a single copy of the phosphoprotein Dishevelled (Dsh) is found. In the genomes of higher organism (including mammals), three genes encoding isoforms of Dishevelled (Dvl1, Dvl2, and Dvl3) are present. In the fly, Dsh functions in the Wnt-sensitive stabilization of intracellular beta-catenin and activation of the Lef/Tcf-sensitive transcriptional response known as the Wnt "canonical" pathway. In the current work we explore the expression of Dishevelleds in mammalian cells and provide an estimate of the relative cellular abundance of each Dvl. In mouse F9 cells, all three Dvls are expressed. Dvl2 constitutes more than 95% of the total pool, the sum of Dvl1 and Dvl3 constituting the remainder. Similarly, Dvl2 constitutes more than 80% of the Dvl1-3 pool in mouse P19 and human HEK 293 cells. siRNA-induced knock-down of individual Dvls was performed using Wnt3a-sensitive canonical pathway in F9 cells as the read-out. Activation of the canonical signaling pathway by Wnt3a was dependent upon the presence of Dvl1, Dvl2, and Dvl3, but to a variable extent. Wnt3a-sensitive canonical transcription was suppressible, by knock-down of Dvl1, Dvl2, or Dvl3. Conversely, the overexpression of any one of the three Dvls individually was found to be capable of promoting Lef/Tcf-sensitive transcriptional activation, in the absence of Wnt3a, i.e., overexpression of Dvl1, Dvl2, or Dvl3 is Wnt3a-mimetic. Graded suppression of individual Dvl isoforms by siRNA was employed to test if the three Dvls could be distinguished from one another with regard to mediation of the canonical pathway. Canonical signaling was most sensitive to changes in the abundance of either Dvl3 or Dvl1. Changes in expression of Dvl2, the most abundant of the three isoforms, resulted in the least effect on canonical signaling. Dvl-based complexes were isolated by pull-downs from whole-cell extracts with isoform-specific antibodies and found to include all three Dvl isoforms. Rescue experiments were conducted in which depletion of either Dvl3 or Dvl1 suppresses Wnt3a activation of the canonical pathway and the ability of a Dvl isoform to rescue the response evaluated. Rescue of Wnt3a-stimulated transcriptional activation in these siRNA-treated cells occurred only by the expression of the very same Dvl isoform depleted by the siRNA. Thus, Dvls appear to function cooperatively as well as uniquely with respect to mediation of Wnt3a-stimulated canonical signaling. The least abundant (Dvl1, 3) plays the most obvious role, whereas the most abundant (Dvl2) plays the least obvious role, suggesting that individual Dvl isoforms in mammals may operate as a network with some features in common and others rather unique.
Figures





Similar articles
-
The cellular story of dishevelleds.Croat Med J. 2014 Oct;55(5):459-67. doi: 10.3325/cmj.2014.55.459. Croat Med J. 2014. PMID: 25358879 Free PMC article. Review.
-
Dishevelled-3 C-terminal His single amino acid repeats are obligate for Wnt5a activation of non-canonical signaling.J Mol Signal. 2010 Nov 23;5:19. doi: 10.1186/1750-2187-5-19. J Mol Signal. 2010. PMID: 21092292 Free PMC article.
-
Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development.PLoS Genet. 2008 Nov;4(11):e1000259. doi: 10.1371/journal.pgen.1000259. Epub 2008 Nov 14. PLoS Genet. 2008. PMID: 19008950 Free PMC article.
-
Dishevelled-2 docks and activates Src in a Wnt-dependent manner.J Cell Sci. 2009 Dec 15;122(Pt 24):4439-51. doi: 10.1242/jcs.051847. Epub 2009 Nov 17. J Cell Sci. 2009. PMID: 19920076 Free PMC article.
-
Dishevelled: The hub of Wnt signaling.Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review.
Cited by
-
LMO2 attenuates tumor growth by targeting the Wnt signaling pathway in breast and colorectal cancer.Sci Rep. 2016 Oct 25;6:36050. doi: 10.1038/srep36050. Sci Rep. 2016. PMID: 27779255 Free PMC article.
-
Anticancer Activity of (S)-5-Chloro-3-((3,5-dimethylphenyl)sulfonyl)-N-(1-oxo-1-((pyridin-4-ylmethyl)amino)propan-2-yl)-1H-indole-2-carboxamide (RS4690), a New Dishevelled 1 Inhibitor.Cancers (Basel). 2022 Mar 7;14(5):1358. doi: 10.3390/cancers14051358. Cancers (Basel). 2022. PMID: 35267666 Free PMC article.
-
The cellular story of dishevelleds.Croat Med J. 2014 Oct;55(5):459-67. doi: 10.3325/cmj.2014.55.459. Croat Med J. 2014. PMID: 25358879 Free PMC article. Review.
-
Dishevelled-3 C-terminal His single amino acid repeats are obligate for Wnt5a activation of non-canonical signaling.J Mol Signal. 2010 Nov 23;5:19. doi: 10.1186/1750-2187-5-19. J Mol Signal. 2010. PMID: 21092292 Free PMC article.
-
Cftr Modulates Wnt/β-Catenin Signaling and Stem Cell Proliferation in Murine Intestine.Cell Mol Gastroenterol Hepatol. 2017 Dec 7;5(3):253-271. doi: 10.1016/j.jcmgh.2017.11.013. eCollection 2018 Mar. Cell Mol Gastroenterol Hepatol. 2017. PMID: 29675451 Free PMC article.
References
-
- Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. - PubMed
-
- Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934–941. - PubMed
-
- Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. - PubMed
-
- Malbon CC. G proteins in development. Nat Rev Mol Cell Biol. 2005;6:689–701. - PubMed
-
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

