Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics
- PMID: 18093917
- PMCID: PMC2410066
- DOI: 10.1073/pnas.0709957104
Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics
Abstract
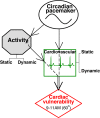
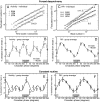
The endogenous circadian pacemaker influences key physiologic functions, such as body temperature and heart rate, and is normally synchronized with the sleep/wake cycle. Epidemiological studies demonstrate a 24-h pattern in adverse cardiovascular events with a peak at approximately 10 a.m. It is unknown whether this pattern in cardiac risk is caused by a day/night pattern of behaviors, including activity level and/or influences from the internal circadian pacemaker. We recently found that a scaling index of cardiac vulnerability has an endogenous circadian peak at the circadian phase corresponding to approximately 10 a.m., which conceivably could contribute to the morning peak in cardiac risk. Here, we test whether this endogenous circadian influence on cardiac dynamics is caused by circadian-mediated changes in motor activity or whether activity and heart rate dynamics are decoupled across the circadian cycle. We analyze high-frequency recordings of motion from young healthy subjects during two complementary protocols that decouple the sleep/wake cycle from the circadian cycle while controlling scheduled behaviors. We find that static activity properties (mean and standard deviation) exhibit significant circadian rhythms with a peak at the circadian phase corresponding to 5-9 p.m. ( approximately 9 h later than the peak in the scale-invariant index of heartbeat fluctuations). In contrast, dynamic characteristics of the temporal scale-invariant organization of activity fluctuations (long-range correlations) do not exhibit a circadian rhythm. These findings suggest that endogenous circadian-mediated activity variations are not responsible for the endogenous circadian rhythm in the scale-invariant structure of heartbeat fluctuations and likely do not contribute to the increase in cardiac risk at approximately 10 a.m.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior.Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18223-7. doi: 10.1073/pnas.0408243101. Epub 2004 Dec 20. Proc Natl Acad Sci U S A. 2004. PMID: 15611476 Free PMC article.
-
Scale-invariant aspects of cardiac dynamics across sleep stages and circadian phases.Conf Proc IEEE Eng Med Biol Soc. 2006;2006:445-8. doi: 10.1109/IEMBS.2006.259760. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17946835 Review.
-
The circadian pacemaker generates similar circadian rhythms in the fractal structure of heart rate in humans and rats.Cardiovasc Res. 2008 Oct 1;80(1):62-8. doi: 10.1093/cvr/cvn150. Epub 2008 Jun 6. Cardiovasc Res. 2008. PMID: 18539630 Free PMC article.
-
A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning.Chronobiol Int. 2012 Jul;29(6):757-68. doi: 10.3109/07420528.2012.674592. Chronobiol Int. 2012. PMID: 22734576
-
Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep.Chronobiol Int. 2000 May;17(3):285-311. doi: 10.1081/cbi-100101049. Chronobiol Int. 2000. PMID: 10841208 Review.
Cited by
-
Integrative Analysis of miRNA and mRNA Expression Profiles Associated With Human Atrial Aging.Front Physiol. 2019 Sep 19;10:1226. doi: 10.3389/fphys.2019.01226. eCollection 2019. Front Physiol. 2019. PMID: 31607954 Free PMC article.
-
Bright Light Increases Alertness and Not Cortisol in Healthy Men: A Forced Desynchrony Study Under Dim and Bright Light (I).J Biol Rhythms. 2022 Aug;37(4):403-416. doi: 10.1177/07487304221096945. Epub 2022 Jun 10. J Biol Rhythms. 2022. PMID: 35686534 Free PMC article.
-
Circadian Rhythms and Measures of CNS/Autonomic Interaction.Int J Environ Res Public Health. 2019 Jul 2;16(13):2336. doi: 10.3390/ijerph16132336. Int J Environ Res Public Health. 2019. PMID: 31269700 Free PMC article. Review.
-
Extracting climate memory using Fractional Integrated Statistical Model: a new perspective on climate prediction.Sci Rep. 2014 Oct 10;4:6577. doi: 10.1038/srep06577. Sci Rep. 2014. PMID: 25300777 Free PMC article.
-
Power considerations for the application of detrended fluctuation analysis in gait variability studies.PLoS One. 2017 Mar 21;12(3):e0174144. doi: 10.1371/journal.pone.0174144. eCollection 2017. PLoS One. 2017. PMID: 28323871 Free PMC article.
References
-
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. N Eng J Med. 1985;313:1315–1322. - PubMed
-
- Goldberg RJ, Brady P, Muller JE, Chen ZY, Degroot M, Zonneveld P, Dalen JE. Am J Cardiol. 1990;66:140–144. - PubMed
-
- Ridker PM, Manson JE, Buring JE, Muller JE, Hennekens CH. Circulation. 1990;82:897–902. - PubMed
-
- Tofler GH, Stone PH, Maclure M, Edelman E, Davis VG, Robertson T, Antman EM, Muller JE. Am J Cardiol. 1990;66:22–27. - PubMed
-
- Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, Hier DB, Wolf PA, Caplan LR, Foulkes MA. Stroke. 1989;20:473–476. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

