Induction of autophagy during extracellular matrix detachment promotes cell survival
- PMID: 18094039
- PMCID: PMC2262959
- DOI: 10.1091/mbc.e07-10-1092
Induction of autophagy during extracellular matrix detachment promotes cell survival
Abstract
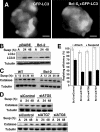
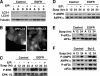
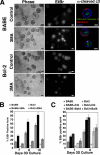
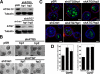
Autophagy has been proposed to promote cell death during lumen formation in three-dimensional mammary epithelial acini because numerous autophagic vacuoles are observed in the dying central cells during morphogenesis. Because these central cells die due to extracellular matrix (ECM) deprivation (anoikis), we have directly interrogated how matrix detachment regulates autophagy. Detachment induces autophagy in both nontumorigenic epithelial lines and in primary epithelial cells. RNA interference-mediated depletion of autophagy regulators (ATGs) inhibits detachment-induced autophagy, enhances apoptosis, and reduces clonogenic recovery after anoikis. Remarkably, matrix-detached cells still exhibit autophagy when apoptosis is blocked by Bcl-2 overexpression, and ATG depletion reduces the clonogenic survival of Bcl-2-expressing cells after detachment. Finally, stable reduction of ATG5 or ATG7 in MCF-10A acini enhances luminal apoptosis during morphogenesis and fails to elicit long-term luminal filling, even when combined with apoptotic inhibition mediated by Bcl-2 overexpression. Thus, autophagy promotes epithelial cell survival during anoikis, including detached cells harboring antiapoptotic lesions.
Figures




References
-
- Chambers A. F., Groom A. C., MacDonald I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. - PubMed
-
- Debnath J., Baehrecke E. H., Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1:e10–e18. - PubMed
-
- Debnath J., Brugge J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer. 2005;5:675–688. - PubMed
-
- Debnath J., Mills K. R., Collins N. L., Reginato M. J., Muthuswamy S. K., Brugge J. S. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

