A unique platform for H-Ras signaling involving clathrin-independent endocytosis
- PMID: 18094044
- PMCID: PMC2262976
- DOI: 10.1091/mbc.e07-08-0841
A unique platform for H-Ras signaling involving clathrin-independent endocytosis
Abstract
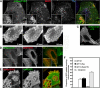
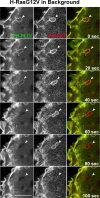
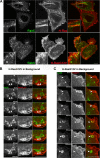
Trafficking of H-Ras was examined to determine whether it can enter cells through clathrin-independent endocytosis (CIE). H-Ras colocalized with the CIE cargo protein, class I major histocompatibility complex, and it was sequestered in vacuoles that formed upon expression of an active mutant of Arf6, Q67L. Activation of Ras, either through epidermal growth factor stimulation or the expression of an active mutant of Ras, G12V, induced plasma membrane ruffling and macropinocytosis, a stimulated form of CIE. Live imaging of cells expressing H-RasG12V and fluorescent protein chimeras with pleckstrin homology domains that recognize specific phosphoinositides showed that incoming macropinosomes contained phosphatidylinositol 4,5-bisphosphate (PIP(2)) and phosphatiylinositol 3,4,5-trisphosphate (PIP(3)). PIP(2) loss from the macropinosome was followed by the recruitment of Rab5, a downstream target of Ras, and then PIP(3) loss. Our studies support a model whereby Ras can signal on macropinosomes that pass through three distinct stages: PIP(2)/PIP(3), PIP(3)/Rab5, and Rab5. Vacuoles that form in cells expressing Arf6Q67L trap Ras signaling in the first stage, recruiting the active form of the Ras effectors extracellular signal-regulated kinase and protein kinase B (Akt) but not Rab5. Arf6 stimulation of macropinocytosis also involves passage through the distinct lipid phases, but recruitment of Akt is not observed.
Figures




References
-
- Amyere M., Mettlen M., Van Der Smissen P., Platek A., Payrastre B., Veithen A., Courtoy P. J. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int. J. Med. Microbiol. 2002;291:487–494. - PubMed
-
- Barbieri M. A., Kohn A. D., Roth R. A., Stahl P. D. Protein kinase B/akt and rab5 mediate Ras activation of endocytosis. J. Biol. Chem. 1998;273:19367–19370. - PubMed
-
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

