Calcium signaling in airway smooth muscle
- PMID: 18094080
- PMCID: PMC2645299
- DOI: 10.1513/pats.200704-047VS
Calcium signaling in airway smooth muscle
Abstract
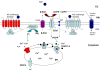
Contractility of airway smooth muscle requires elevation of intracellular calcium concentration. Under resting conditions, airway smooth muscle cells maintain a relatively low intracellular calcium concentration, and activation of the surface receptors by contractile agonists results in an elevation of intracellular calcium, culminating in contraction of the cell. The pattern of elevation of intracellular calcium brought about by agonists is a dynamic process and involves the coordinated activities of ion channels located in the plasma membrane and the sarcoplasmic reticulum. Among the signaling molecules involved in this dynamic calcium regulation in airway smooth muscle cells are inositol 1,4,5-trisphosphate and cyclic ADP-ribose, which mobilize calcium from the sarcoplasmic reticulum by acting via the inositol 1,4,5-trisphosphate and ryanodine receptors, respectively. In addition, calcium influx from the extracellular space is critical for the repletion of the intracellular calcium stores during activation of the cells by agonists. Calcium influx can occur via voltage- and receptor-gated channels in the plasma membrane, as well as by influx that is triggered by depletion of the intracellular stores (i.e., store-operated calcium entry mechanism). Transient receptor potential proteins appear to mediate the calcium influx via receptor- and store-operated channels. Recent studies have shown that proinflammatory cytokines regulate the expression and activity of the pathways involved in intracellular calcium regulation, thereby contributing to airway smooth muscle cell hyperresponsiveness. In this review, we will discuss the specific roles of cyclic ADP-ribose/ryanodine receptor channels and transient receptor potential channels in the regulation of intracellular calcium in airway smooth muscle cells.
Figures

Similar articles
-
CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2005 May;288(5):L773-88. doi: 10.1152/ajplung.00217.2004. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15821018 Review.
-
The relative contributions of store-operated and voltage-gated Ca2+ channels to the control of Ca2+ oscillations in airway smooth muscle.J Physiol. 2017 May 15;595(10):3129-3141. doi: 10.1113/JP272996. Epub 2016 Sep 21. J Physiol. 2017. PMID: 27502470 Free PMC article.
-
Maintenance of intracellular Ca2+ basal concentration in airway smooth muscle (Review).Int J Mol Med. 2018 Dec;42(6):2998-3008. doi: 10.3892/ijmm.2018.3910. Epub 2018 Oct 2. Int J Mol Med. 2018. PMID: 30280184 Free PMC article. Review.
-
Store-operated calcium entry is required for sustained contraction and Ca2+ oscillations of airway smooth muscle.J Physiol. 2017 May 15;595(10):3203-3218. doi: 10.1113/JP272694. Epub 2016 Aug 2. J Physiol. 2017. PMID: 27396568 Free PMC article.
-
Altered airway responsiveness in CD38-deficient mice.Am J Respir Cell Mol Biol. 2005 Feb;32(2):149-56. doi: 10.1165/rcmb.2004-0243OC. Epub 2004 Nov 19. Am J Respir Cell Mol Biol. 2005. PMID: 15557017
Cited by
-
A multi-scale approach to airway hyperresponsiveness: from molecule to organ.Front Physiol. 2012 Jun 11;3:191. doi: 10.3389/fphys.2012.00191. eCollection 2012. Front Physiol. 2012. PMID: 22701430 Free PMC article.
-
Airway smooth muscle in airway reactivity and remodeling: what have we learned?Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L912-33. doi: 10.1152/ajplung.00259.2013. Epub 2013 Oct 18. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24142517 Free PMC article. Review.
-
Airway smooth muscle in contractility and remodeling of asthma: potential drug target mechanisms.Expert Opin Ther Targets. 2023 Jan;27(1):19-29. doi: 10.1080/14728222.2023.2177533. Epub 2023 Feb 13. Expert Opin Ther Targets. 2023. PMID: 36744401 Free PMC article.
-
Development of a QPatch-Automated Electrophysiology Assay for Identifying TMEM16A Small-Molecule Inhibitors.Assay Drug Dev Technol. 2020 Apr;18(3):134-147. doi: 10.1089/adt.2019.962. Assay Drug Dev Technol. 2020. PMID: 32319819 Free PMC article.
-
In vitro and in silico studies of 8(17),12E,14-labdatrien-18-oic acid in airways smooth muscle relaxation: new molecular insights about its mechanism of action.Naunyn Schmiedebergs Arch Pharmacol. 2021 May;394(5):885-902. doi: 10.1007/s00210-020-02010-0. Epub 2020 Nov 18. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33205250
References
-
- Shieh CC, Petrini MF, Dwyer TM, Farley JM. Concentration-dependence of acetylcholine-induced changes in calcium and tension in swine trachealis. J Pharmacol Exp Ther 1991;256:141–148. - PubMed
-
- Sims SM, Jiao Y, Zheng ZG. Intracellular calcium stores in isolated tracheal smooth muscle cells. Am J Physiol 1996;271:L300–L309. - PubMed
-
- Murray RK, Bennett CF, Fluharty SJ, Kotlikoff MI. Mechanism of phorbol ester inhibition of histamine-induced IP3 formation in cultured airway smooth muscle. Am J Physiol 1989;257:L209–L216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
