Sequential analysis of Anaplasma phagocytophilum msp2 transcription in murine and equine models of human granulocytic anaplasmosis
- PMID: 18094110
- PMCID: PMC2268257
- DOI: 10.1128/CVI.00417-07
Sequential analysis of Anaplasma phagocytophilum msp2 transcription in murine and equine models of human granulocytic anaplasmosis
Abstract
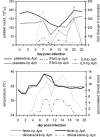
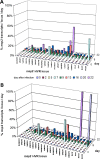
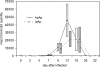
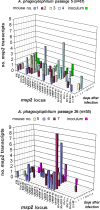
Anaplasma phagocytophilum causes human granulocytic anaplasmosis by inducing immunopathologic responses. Its immunodominant Msp2 protein is encoded by a family of >100 paralogs. Msp2 (msp2) expression modulates in the absence of immune pressure, and prolonged in vitro passage modulates in vivo virulence. Because programmed MSP2 expression occurs in Anaplasma marginale, we hypothesized a similar event in A. phagocytophilum in vivo, with specific Msp2 expression triggering immunopathologic injury or clinical manifestations of disease. We examined msp2 transcripts in 11 B6 mice and 6 horses inoculated with low- or high-passage A. phagocytophilum Webster strain. Blood was sequentially obtained through 3 weeks postinfection for msp2 reverse transcription-PCR. Horses were additionally assessed for clinical manifestations, seroconversion, complete blood count, blood chemistry, and cytokine gene transcription. In both species, there was no consistent emergence of msp2 transcripts, and all 22 msp2 variants were detected in both passage groups. Clinical severity was much higher for high-passage-infected than for low-passage-infected horses, preceded by higher levels of blood gamma interferon transcription on day 7. Antibody was first detected on day 7, and all horses seroconverted by day 22, with a trend toward lower antibody titers in low-passage-infected animals. Leukocyte and platelet counts were similar between experimental groups except on day 13, when low-passage-infected animals had more profound thrombocytopenia. These findings corroborate studies with mice, where msp2 diversity did not explain differences in hepatic histopathology, but differ from the paradigm of low-passage A. phagocytophilum causing more significant clinical illness. Alteration in transcription of msp2 has no bearing on clinical disease in horses, suggesting the existence of a separate proinflammatory component differentially expressed with changing in vitro passage.
Figures


Similar articles
-
Msp2 variation in Anaplasma phagocytophilum in vivo does not stimulate T cell immune responses or interferon-gamma production.FEMS Immunol Med Microbiol. 2007 Apr;49(3):374-86. doi: 10.1111/j.1574-695X.2007.00214.x. Epub 2007 Feb 7. FEMS Immunol Med Microbiol. 2007. PMID: 17286796
-
Anaplasma phagocytophilum has a functional msp2 gene that is distinct from p44.Infect Immun. 2004 Jul;72(7):3883-9. doi: 10.1128/IAI.72.7.3883-3889.2004. Infect Immun. 2004. PMID: 15213131 Free PMC article.
-
Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum.Infect Immun. 2003 Apr;71(4):1706-18. doi: 10.1128/IAI.71.4.1706-1718.2003. Infect Immun. 2003. PMID: 12654783 Free PMC article.
-
Equine granulocytic anaplasmosis.Res Vet Sci. 2013 Oct;95(2):316-20. doi: 10.1016/j.rvsc.2013.05.010. Epub 2013 Jun 19. Res Vet Sci. 2013. PMID: 23790982 Review.
-
Human granulocytic anaplasmosis and Anaplasma phagocytophilum.Emerg Infect Dis. 2005 Dec;11(12):1828-34. doi: 10.3201/eid1112.050898. Emerg Infect Dis. 2005. PMID: 16485466 Free PMC article. Review.
Cited by
-
Dexamethasone-induced cytokine changes associated with diminished disease severity in horses infected with Anaplasma phagocytophilum.Clin Vaccine Immunol. 2011 Nov;18(11):1962-8. doi: 10.1128/CVI.05034-11. Epub 2011 Aug 31. Clin Vaccine Immunol. 2011. PMID: 21880854 Free PMC article.
-
Molecular characterization of Msp2/P44 of Anaplasma phagocytophilum isolated from infected patients and Haemaphysalis longicornis in Laizhou Bay, Shandong Province, China.PLoS One. 2013 Oct 22;8(10):e78189. doi: 10.1371/journal.pone.0078189. eCollection 2013. PLoS One. 2013. PMID: 24167608 Free PMC article. Clinical Trial.
-
An efficient microinjection method to generate human anaplasmosis agent Anaplasma phagocytophilum-infected ticks.Sci Rep. 2020 Sep 29;10(1):15994. doi: 10.1038/s41598-020-73061-9. Sci Rep. 2020. PMID: 32994497 Free PMC article.
-
The biological basis of severe outcomes in Anaplasma phagocytophilum infection.FEMS Immunol Med Microbiol. 2012 Feb;64(1):13-20. doi: 10.1111/j.1574-695X.2011.00909.x. Epub 2011 Dec 19. FEMS Immunol Med Microbiol. 2012. PMID: 22098465 Free PMC article. Review.
-
Differential Susceptibility of Male Versus Female Laboratory Mice to Anaplasma phagocytophilum Infection.Trop Med Infect Dis. 2018 Jul 23;3(3):78. doi: 10.3390/tropicalmed3030078. Trop Med Infect Dis. 2018. PMID: 30274474 Free PMC article.
References
-
- Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. - PubMed
-
- Barbet, A. F., P. F. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. - PMC - PubMed
-
- Borjesson, D. L., and S. W. Barthold. 2002. The mouse as a model for investigation of human granulocytic ehrlichiosis: current knowledge and future directions. Comp. Med. 52:403-413. - PubMed
-
- Borjesson, D. L., J. L. Brazzell, and R. Feferman. 2005. Platelet dysfunction after association with Anaplasma phagocytophilum in vitro. Ann. N.Y. Acad. Sci. 1063:413-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

