The DNase of gammaherpesviruses impairs recognition by virus-specific CD8+ T cells through an additional host shutoff function
- PMID: 18094150
- PMCID: PMC2258936
- DOI: 10.1128/JVI.01946-07
The DNase of gammaherpesviruses impairs recognition by virus-specific CD8+ T cells through an additional host shutoff function
Abstract
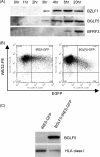
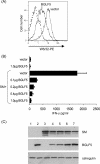
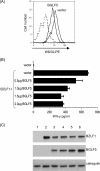
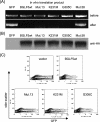
The DNase/alkaline exonuclease (AE) genes are well conserved in all herpesvirus families, but recent studies have shown that the AE proteins of gammaherpesviruses such as Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) exhibit an additional function which shuts down host protein synthesis. One correlate of this additional shutoff function is that levels of cell surface HLA molecules are downregulated, raising the possibility that shutoff/AE genes of gammaherpesviruses might contribute to viral immune evasion. In this study, we show that both BGLF5 (EBV) and SOX (KSHV) shutoff/AE proteins do indeed impair the ability of virus-specific CD8+ T-cell clones to recognize endogenous antigen via HLA class I. Random mutagenesis of the BGLF5 gene enabled us to genetically separate the shutoff and AE functions and to demonstrate that the shutoff function was the critical factor determining whether BGLF5 mutants can impair T-cell recognition. These data provide further evidence that EBV has multiple mechanisms to modulate HLA class I-restricted T-cell responses, thus enabling the virus to replicate and persist in the immune-competent host.
Figures



References
-
- Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9395-404. - PubMed
-
- Barnstable, C. J., W. F. Bodmer, G. Brown, G. Galfre, C. Milstein, A. F. Williams, and A. Ziegler. 1978. Production of a monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens: new tools for genetic analysis. Cell 149-20. - PubMed
-
- Cook, I. D., F. Shanahan, and P. J. Farrell. 1994. Epstein-Barr virus SM protein. Virology 205217-227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

