Jaagsiekte sheep retrovirus utilizes a pH-dependent endocytosis pathway for entry
- PMID: 18094164
- PMCID: PMC2258929
- DOI: 10.1128/JVI.01853-07
Jaagsiekte sheep retrovirus utilizes a pH-dependent endocytosis pathway for entry
Abstract
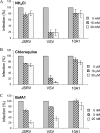
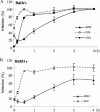
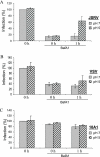
Using Moloney murine leukemia virus pseudovirions bearing the envelope protein of Jaagsiekte sheep retrovirus (JSRV), we report here that entry was weakly inhibited by lysosomotropic agents but was profoundly blocked by bafilomycin A1 (BafA1). Kinetics studies revealed that JSRV entry is a slow process and was substantially blocked by a dominant-negative mutant of dynamin. Interestingly, a low-pH pulse overcame the BafA1 block to JSRV infection, although this occurred only if virus-bound cells were preincubated at 37 degrees C, consistent with a very early entry event such as endocytosis being required before the low-pH-dependent step occurs. Moreover, JSRV pseudovirions were resistant to low-pH inactivation. Altogether, this study reveals that JSRV utilizes a pH-dependent, dynamin-associated endocytosis pathway for entry that differs from the classical pH-dependent entry pathway of vesicular stomatitis virus.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

