Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons
- PMID: 18094230
- PMCID: PMC6673524
- DOI: 10.1523/JNEUROSCI.3520-07.2007
Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons
Abstract
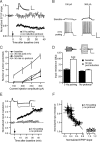
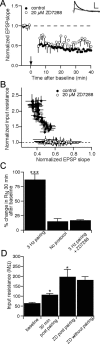
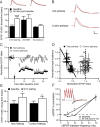
Bidirectional changes in synaptic strength are the proposed cellular correlate for information storage in the brain. Plasticity of intrinsic excitability, however, may also be critical for regulating the firing of neurons during mnemonic tasks. We demonstrated previously that the induction long-term potentiation was accompanied by a persistent decrease in CA1 pyramidal neuron excitability (Fan et al., 2005). We show here that induction of long-term depression (LTD) by 3 Hz pairing of back-propagating action potentials with Schaffer collateral EPSPs was accompanied by an overall increase in CA1 neuronal excitability. This increase was observed as an increase in the number of action potentials elicited by somatic current injection and was caused by an increase in neuronal input resistance. After LTD, voltage sag during hyperpolarizing current injections and subthreshold resonance frequency were decreased. All changes were blocked by ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride), suggesting that a physiological loss of dendritic h-channels was responsible for the increase in excitability. Furthermore, block of group 1 metabotropic glutamate receptors (mGluRs) or protein kinase C prevented the increase in excitability, whereas the group 1 mGluR agonist DHPG [(RS)-3,5-dihydroxyphenylglycine] mimicked the effects. We conclude that 3 Hz synaptic stimulation downregulates I(h) via activation of group 1 mGluRs and subsequent stimulation of protein kinase C. We propose these changes as part of a homeostatic and bidirectional control mechanism for intrinsic excitability during learning.
Figures






References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. - PubMed
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. - PubMed
-
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
