Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake
- PMID: 18094243
- PMCID: PMC2975593
- DOI: 10.1523/JNEUROSCI.3217-07.2007
Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake
Abstract
The hypocretin (HCRT) neurons are located only in the perifornical area of the lateral hypothalamus and heavily innervate the cholinergic neurons in the basal forebrain (BF), histamine neurons in the tuberomammillary nucleus (TMN), and the noradrenergic locus ceruleus (LC) neurons, three neuronal populations that have traditionally been implicated in regulating arousal. Based on the innervation, HCRT neurons may regulate arousal by driving these downstream arousal neurons. Here, we directly test this hypothesis by a simultaneous triple lesion of these neurons using three saporin-conjugated neurotoxins. Three weeks after lesion, the daily levels of wake were not changed in rats with double or triple lesions, although rats with triple lesions were asleep more during the light-to-dark transition period. The double- and triple-lesioned rats also had more stable sleep architecture compared with nonlesioned saline rats. These results suggest that the cholinergic BF, TMN, and LC neurons jointly modulate arousal at a specific circadian time, but they are not essential links in the circuitry responsible for daily levels of wake, as traditionally hypothesized.
Figures

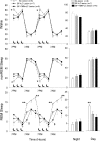
 p < 0.05 versus double lesion (post hoc after one-way ANOVA).
p < 0.05 versus double lesion (post hoc after one-way ANOVA).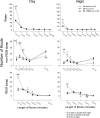
References
-
- Blanco-Centurion CA, Shiromani A, Winston E, Shiromani PJ. Effects of hypocretin-1 in 192-IgG-saporin-lesioned rats. Eur J Neurosci. 2006b;24:2084–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
