ARP/wARP and molecular replacement: the next generation
- PMID: 18094467
- PMCID: PMC2394809
- DOI: 10.1107/S0907444907047580
ARP/wARP and molecular replacement: the next generation
Abstract
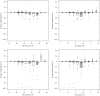
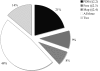
Automatic iterative model (re-)building, as implemented in ARP/wARP and its new control system flex-wARP, is particularly well suited to follow structure solution by molecular replacement. More than 100 molecular-replacement solutions automatically solved by the BALBES software were submitted to three standard protocols in flex-wARP and the results were compared with final models from the PDB. Standard metrics were gathered in a systematic way and enabled the drawing of statistical conclusions on the advantages of each protocol. Based on this analysis, an empirical estimator was proposed that predicts how good the final model produced by flex-wARP is likely to be based on the experimental data and the quality of the molecular-replacement solution. To introduce the differences between the three flex-wARP protocols (keeping the complete search model, converting it to atomic coordinates but ignoring atom identities or using the electron-density map calculated from the molecular-replacement solution), two examples are also discussed in detail, focusing on the evolution of the models during iterative rebuilding. This highlights the diversity of paths that the flex-wARP control system can employ to reach a nearly complete and accurate model while actually starting from the same initial information.
Figures




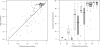
References
-
- Banci, L. et al. (2006). Acta Cryst. D62, 1208–1217. - PubMed
-
- Cohen, S. X., Morris, R. J., Fernandez, F. J., Ben Jelloul, M., Kakaris, M., Parthasarathy, V., Lamzin, V. S., Kleywegt, G. J. & Perrakis, A. (2004). Acta Cryst. D60, 2222–2229. - PubMed
-
- Cowtan, K. (2006). Acta Cryst. D62, 1002–1011. - PubMed
-
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. - PubMed
-
- Ioerger, T. R. & Sacchettini, J. C. (2003). Methods Enzymol.374, 244–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

