Normophosphatemic familial tumoral calcinosis is caused by deleterious mutations in SAMD9, encoding a TNF-alpha responsive protein
- PMID: 18094730
- PMCID: PMC3169318
- DOI: 10.1038/sj.jid.5701203
Normophosphatemic familial tumoral calcinosis is caused by deleterious mutations in SAMD9, encoding a TNF-alpha responsive protein
Abstract
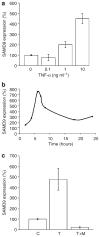
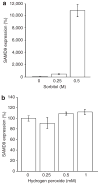
Normophosphatemic familial tumoral calcinosis (NFTC) is an autosomal recessive disorder characterized by calcium deposition in skin and mucosae and associated with unremitting pain and life-threatening skin infections. A homozygous missense mutation (p.K1495E), resulting in SAMD9 protein degradation, was recently shown to cause NFTC in five families of Jewish-Yemenite origin. In this study, we evaluated another Jewish-Yemenite NFTC kindred. All patients were compound heterozygous for two mutations in SAMD9: K1495E and a previously unreported nonsense mutation, R344X, predicted to result in a markedly truncated molecule. Screening of unaffected population-matched controls revealed heterozygosity for K1495E and R344X only in individuals of Jewish-Yemenite ancestry, but not in more than 700 control samples of other origins, including 93 non-Jewish Yemenite. These data may be suggestive of positive selection, considering the rarity of NFTC and the small size of the Jewish-Yemenite population; alternatively, they may reflect genetic drift or the effect of a population-specific modifier trait. Calcifications in NFTC generally develop over areas subjected to repeated trauma and are associated with marked inflammatory manifestations, indicating that SAMD9 may play a role in the inflammatory response to tissue injury. We therefore assessed the effect of cellular stress and tumor necrosis factor-alpha (TNF-alpha), a potent pro-inflammatory cytokine, on SAMD9 gene expression. Whereas exogenous hydrogen peroxide and heat shock did not affect SAMD9 transcription, osmotic shock was found to markedly upregulate SAMD9 expression. In addition, incubation of endothelial cells with TNF-alpha caused a dose-related, p38-dependant increase in SAMD9 expression. These data link NFTC and SAMD9 to the TNF-alpha signaling pathway, suggesting a role for this system in the regulation of extra-osseous calcification.
Conflict of interest statement
The authors state no conflict of interest.
Figures


References
-
- Alpert D, Schwenger P, Han J, Vilcek J. Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit tumor necrosis factor-induced IkappaB phosphorylation and NF-kappaB activation. J Biol Chem. 1999;274:22176–83. - PubMed
-
- Antonarakis SE, Beckmann JS. Mendelian disorders deserve more attention. Nat Rev. 2006;7:277–82. - PubMed
-
- Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, et al. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metabol. 2005;90:5523–7. - PubMed
-
- Atzeni F, Sarzi-Puttini P, Bevilacqua M. Calcium deposition and associated chronic diseases (atherosclerosis, diffuse idiopathic skeletal hyperostosis, and others) Rheum Dis Clin North Am. 2006;32:413–26. viii. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

