Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria
- PMID: 18094751
- PMCID: PMC2147073
- DOI: 10.1371/journal.pone.0001343
Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria
Abstract
Background: Mycolic acids are a complex mixture of branched, long-chain fatty acids, representing key components of the highly hydrophobic mycobacterial cell wall. Pathogenic mycobacteria carry mycolic acid sub-types that contain cyclopropane rings. Double bonds at specific sites on mycolic acid precursors are modified by the action of cyclopropane mycolic acid synthases (CMASs). The latter belong to a family of S-adenosyl-methionine-dependent methyl transferases, of which several have been well studied in Mycobacterium tuberculosis, namely, MmaA1 through A4, PcaA and CmaA2. Cyclopropanated mycolic acids are key factors participating in cell envelope permeability, host immunomodulation and persistence of M. tuberculosis. While several antitubercular agents inhibit mycolic acid synthesis, to date, the CMASs have not been shown to be drug targets.
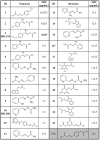
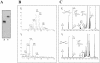
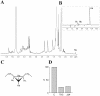
Methodology/principle findings: We have employed various complementary approaches to show that the antitubercular drug, thiacetazone (TAC), and its chemical analogues, inhibit mycolic acid cyclopropanation. Dramatic changes in the content and ratio of mycolic acids in the vaccine strain Mycobacterium bovis BCG, as well as in the related pathogenic species Mycobacterium marinum were observed after treatment with the drugs. Combination of thin layer chromatography, mass spectrometry and Nuclear Magnetic Resonance (NMR) analyses of mycolic acids purified from drug-treated mycobacteria showed a significant loss of cyclopropanation in both the alpha- and oxygenated mycolate sub-types. Additionally, High-Resolution Magic Angle Spinning (HR-MAS) NMR analyses on whole cells was used to detect cell wall-associated mycolates and to quantify the cyclopropanation status of the cell envelope. Further, overexpression of cmaA2, mmaA2 or pcaA in mycobacteria partially reversed the effects of TAC and its analogue on mycolic acid cyclopropanation, suggesting that the drugs act directly on CMASs.
Conclusions/significance: This is a first report on the mechanism of action of TAC, demonstrating the CMASs as its cellular targets in mycobacteria. The implications of this study may be important for the design of alternative strategies for tuberculosis treatment.
Conflict of interest statement
Figures



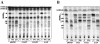
References
-
- Dorman SE, Chaisson RE. From magic bullets back to the magic mountain: the rise of extensively drug-resistant tuberculosis. Nat Med. 2007;13:295–298. - PubMed
-
- Raviglione MC, Smith IM. XDR tuberculosis–implications for global public health. N Engl J Med. 2007;356:656–659. - PubMed
-
- Prevention CfDCa. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs–worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. - PubMed
-
- Davidson PT, Le HQ. Drug treatment of tuberculosis–1992. Drugs. 1992;43:651–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

