Biomolecular gradients in cell culture systems
- PMID: 18094760
- PMCID: PMC3848882
- DOI: 10.1039/b711887b
Biomolecular gradients in cell culture systems
Abstract
Biomolecule gradients have been shown to play roles in a wide range of biological processes including development, inflammation, wound healing, and cancer metastasis. Elucidation of these phenomena requires the ability to expose cells to biomolecule gradients that are quantifiable, controllable, and mimic those that are present in vivo. Here we review the major biological phenomena in which biomolecule gradients are employed, traditional in vitro gradient-generating methods developed over the past 50 years, and new microfluidic devices for generating gradients. Microfluidic gradient generators offer greater levels of precision, quantitation, and spatiotemporal gradient control than traditional methods, and may greatly enhance our understanding of many biological phenomena. For each method, we outline the salient features, capabilities, and applications.
Figures


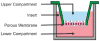


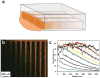

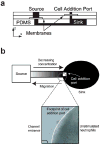


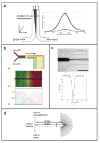
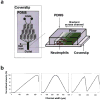
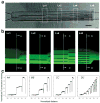




References
-
- Gilbert SF. Developmental Biology. 8. xviii. Sinauer Associates, Inc. Publishers; Sunderland, MA: 2006. p. 817.
-
- Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133(3):385–394. - PubMed
-
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295(5563):2231–2235. - PubMed
-
- Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu Rev Neurosci. 1995;18:497–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

