Lamellarins and related pyrrole-derived alkaloids from marine organisms
- PMID: 18095718
- PMCID: PMC4928200
- DOI: 10.1021/cr078199m
Lamellarins and related pyrrole-derived alkaloids from marine organisms
Erratum in
- Chem Rev. 2010 Jun 9;110(6):3850
Figures
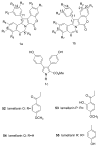
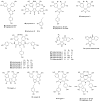
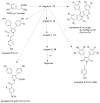
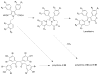

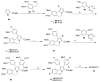
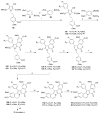
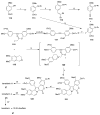
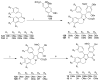
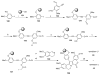
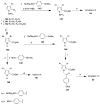

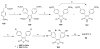
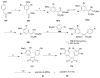
References
-
- Andersen RJ, Faulkner DJ, He C-H, Van Duyne GD, Clardy J. J Am Chem Soc. 1985;107:5492.
-
- Cantrell CL, Groweiss A, Gustafson KR, Boyd MR. Nat Prod Lett. 1999;14:39.
-
- Lindquist N, Fenical W, Van Duyne GD, Clardy J. J Org Chem. 1988;53:4570.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

