The effect of oral sodium acetate administration on plasma acetate concentration and acid-base state in horses
- PMID: 18096070
- PMCID: PMC2241837
- DOI: 10.1186/1751-0147-49-38
The effect of oral sodium acetate administration on plasma acetate concentration and acid-base state in horses
Abstract
Aim: Sodium acetate (NaAcetate) has received some attention as an alkalinizing agent and possible alternative energy source for the horse, however the effects of oral administration remain largely unknown. The present study used the physicochemical approach to characterize the changes in acid-base status occurring after oral NaAcetate/acetic acid (NAA) administration in horses.
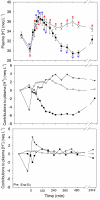
Methods: Jugular venous blood was sampled from 9 exercise-conditioned horses on 2 separate occasions, at rest and for 24 h following a competition exercise test (CET) designed to simulate the speed and endurance test of 3-day event. Immediately after the CETs horses were allowed water ad libitum and either: 1) 8 L of a hypertonic NaAcetate/acetic acid solution via nasogastric tube followed by a typical hay/grain meal (NAA trial); or 2) a hay/grain meal alone (Control trial).
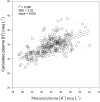
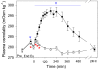
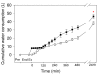
Results: Oral NAA resulted in a profound plasma alkalosis marked by decreased plasma [H+] and increased plasma [TCO2] and [HCO3-] compared to Control. The primary contributor to the plasma alkalosis was an increased [SID], as a result of increased plasma [Na+] and decreased plasma [Cl-]. An increased [Atot], due to increased [PP] and a sustained increase in plasma [acetate], contributed a minor acidifying effect.
Conclusion: It is concluded that oral NaAcetate could be used as both an alkalinizing agent and an alternative energy source in the horse.
Figures




References
-
- Schott HC, Hinchcliff KW. Fluids electrolytes and bicarbonate. In: Hinchcliff KW, Sams RA, editor. Vet Clin North Am: equine practice – drug use in performance horses. Philadelphia: WB Saunders; 1993. pp. 577–604. - PubMed
-
- Heigenhauser GJ, Jones NL. Bicarbonate loading. In: Lamb DR, Williams MH, editor. Perspectives in exercise science and sports medicine, Ergogenics – enhancement of performance exercise and sport. Vol. 4. Dubuque, IA: Brown and Benchmark; 1991. pp. 183–221.
-
- Lloyd DR, Rose RJ. Effects of several alkalinizing agents on acid-base balance in horses. Proceedings of the 11th International Conference of Racing Analysts and Veterinarians. 1996. pp. 104–110.
-
- Kline K, Frey LP, Foreman JH, Lyman JT. Effects of intravenous sodium bicarbonate and sodium acetate on equine acid-base status. J Eq Vet Sci. 2005;25:349–354. doi: 10.1016/j.jevs.2005.07.003. - DOI
-
- Stewart PA. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1441–1461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

