A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia
- PMID: 18096137
- PMCID: PMC2693008
- DOI: 10.1016/j.biopsych.2007.11.002
A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia
Abstract
Background: Individuals with schizophrenia smoke at higher rates (58%-88%) than the general population (approximately 22%), and have difficulty quitting. We determined whether the combination of sustained-release (SR) bupropion (BUP) with the transdermal nicotine patch (TNP) was well-tolerated and superior to placebo (PLO)+TNP for smoking cessation in schizophrenia.
Methods: A 10-week, double-blind, placebo-controlled trial of BUP (300 mg/day) in combination with TNP (21 mg/24h) for 58 outpatient smokers with schizophrenia was conducted. Primary outcome measures were continuous smoking abstinence in the last 4 weeks of the trial (Days 43-70) and 7-day point prevalence abstinence at 6 months post-target quit date (TQD) (week 26).
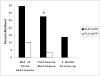
Results: Smokers assigned to the BUP+TNP group (n = 29) were more likely to achieve continuous smoking abstinence (8/29, 27.6%) than the PLO+TNP group (n = 29, 1/29, 3.4%) [Fisher's Exact Test, p < .05]; at 6-months post-TQD, 4/29 (13.8%) versus 0/29 (0.0%) achieved 7-day point prevalence smoking abstinence (p = .11). Neither bupropion SR nor smoking abstinence significantly altered the positive or negative symptoms of schizophrenia. The combination was well-tolerated in smokers with schizophrenia.
Conclusions: Combination therapy with bupropion SR+TNP versus placebo+TNP is well-tolerated and significantly improved short-term smoking abstinence in smokers with schizophrenia.
Trial registration: ClinicalTrials.gov NCT00124683.
Figures
References
-
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence and schizophrenia: clinical phenomenon and laboratory findings. Am J Psychiatry. 1998;155:1490–1501. - PubMed
-
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Res. 2005;76:135–157. - PubMed
-
- Ziedonis DM, Williams JM. Management of smoking in people with psychiatric disorders. Curr Opin Psychiatry. 2003;16:305–315.
-
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. J Am Med Assoc. 2000;284:2606–2610. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

